For Questions 20-24 consider the following scenario: A 0.8390 g of an unknown sample containing chloride was dissolved in water and reacted with an excess of AgNO3 solution. The following data was collected from the gravimetric analysis: Mass of Unknown Sample 0.8390 g Mass of Filter Paper and Precipitate 3.2174 g Mass of Filter Paper 1.0230 g It was determined in the previous question that 2.1944 g (or 0.015308 mol) of AgCl precipitated. Calculate the moles of chloride in the precipitate.
For Questions 20-24 consider the following scenario: A 0.8390 g of an unknown sample containing chloride was dissolved in water and reacted with an excess of AgNO3 solution. The following data was collected from the gravimetric analysis: Mass of Unknown Sample 0.8390 g Mass of Filter Paper and Precipitate 3.2174 g Mass of Filter Paper 1.0230 g It was determined in the previous question that 2.1944 g (or 0.015308 mol) of AgCl precipitated. Calculate the moles of chloride in the precipitate.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 55QAP: A common method for determining how much chloride ion is present in a sample is to precipitate the...
Related questions
Question
help
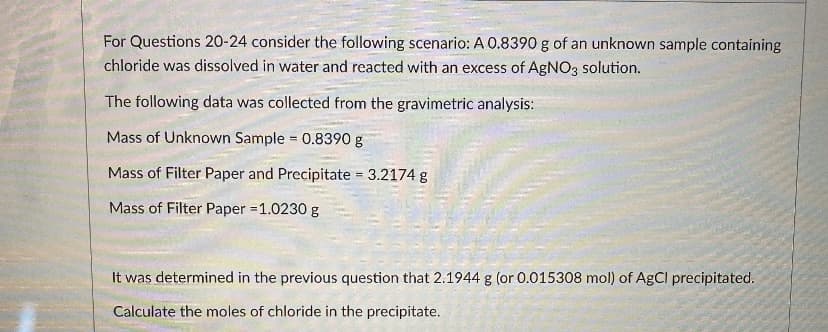
Transcribed Image Text:For Questions 20-24 consider the following scenario: A 0.8390 g of an unknown sample containing
chloride was dissolved in water and reacted with an excess of AgNO3 solution.
The following data was collected from the gravimetric analysis:
Mass of Unknown Sample 0.8390 g
Mass of Filter Paper and Precipitate = 3.2174 g
Mass of Filter Paper =1.0230 g
It was determined in the previous question that 2.1944 g (or 0.015308 mol) of AgCI precipitated.
Calculate the moles of chloride in the precipitate.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
