aDraw and explain the detailed mechanisim of the reaction bWill the reaction work equally well with primary, secondary and tertialry alkyl halides? explain cWhy should the experimental set up be dismantled immediately after reaction is done? dWhat is the limiting reagent of this reaction and why?
Background:
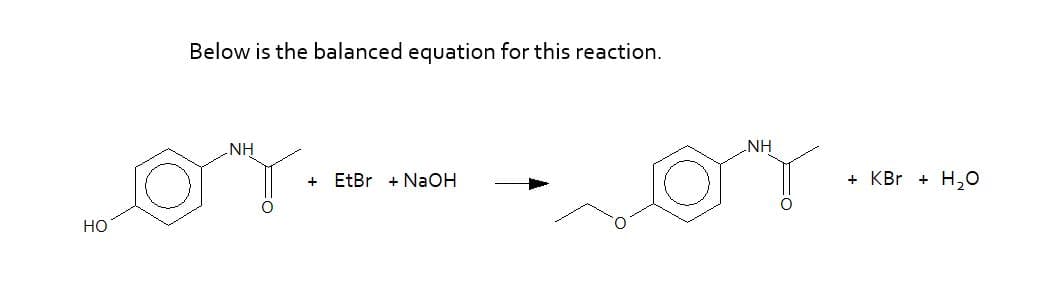
Synthesize phenacetin from acetaminophen (Tylenol) via a Williamson ether synthesis.The Williamson ether synthesis is one of the simplest methods used to make ethers. The reaction is named after Alexander Williamson, who discovered the reaction in 1850. In this reaction, an alkoxide anion reacts with an alkyl halide via a substitution reaction.
RO-Na+ + R` – X → R-O-R` + NaX
Alkoxides are strong nucleophiles that tend to react via an SN2 mechanism. As a result, the reaction works best with primary
In a traditional Williamson ether synthesis, the alkoxide is generated by reacting sodium hydride (NaH) with an alcohol.
ROH + NaH → RO-Na+ + H2
While this reaction is very efficient, it does pose a fire hazard, as it is possible for hydrogen gas to ignite.
In this lab, the alkoxide will be created using sodium hydroxide (NaOH). This is a much safer option, eliminating a substantial portion of the fire hazard. Most alcohols could not efficiently deprotonated by hydroxide, owing to the similar acidities of water and alcohols. However, our reaction requires removing a phenolic (ArOH) proton which is much more acidic than protons from alkyl-substituted alcohols.
|
compound |
pKa |
|
H2 |
36 |
|
ROH (aliphatic) |
15-17 |
|
H2O |
15.7 |
|
p-AcNH-C6H4-OH |
9.5 |
ArOH + OH- ⇄ ArO- + H2O
pKa = 9.5 15.7
The equilibrium favors the right side of the equation. We will use an excess of sodium hydroxide and adequate reflux time to ensure the alkoxide is created and then reacts with bromoethane
Questions
aDraw and explain the detailed mechanisim of the reaction
bWill the reaction work equally well with primary, secondary and tertialry alkyl halides? explain
cWhy should the experimental set up be dismantled immediately after reaction is done?
dWhat is the limiting reagent of this reaction and why?
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images









