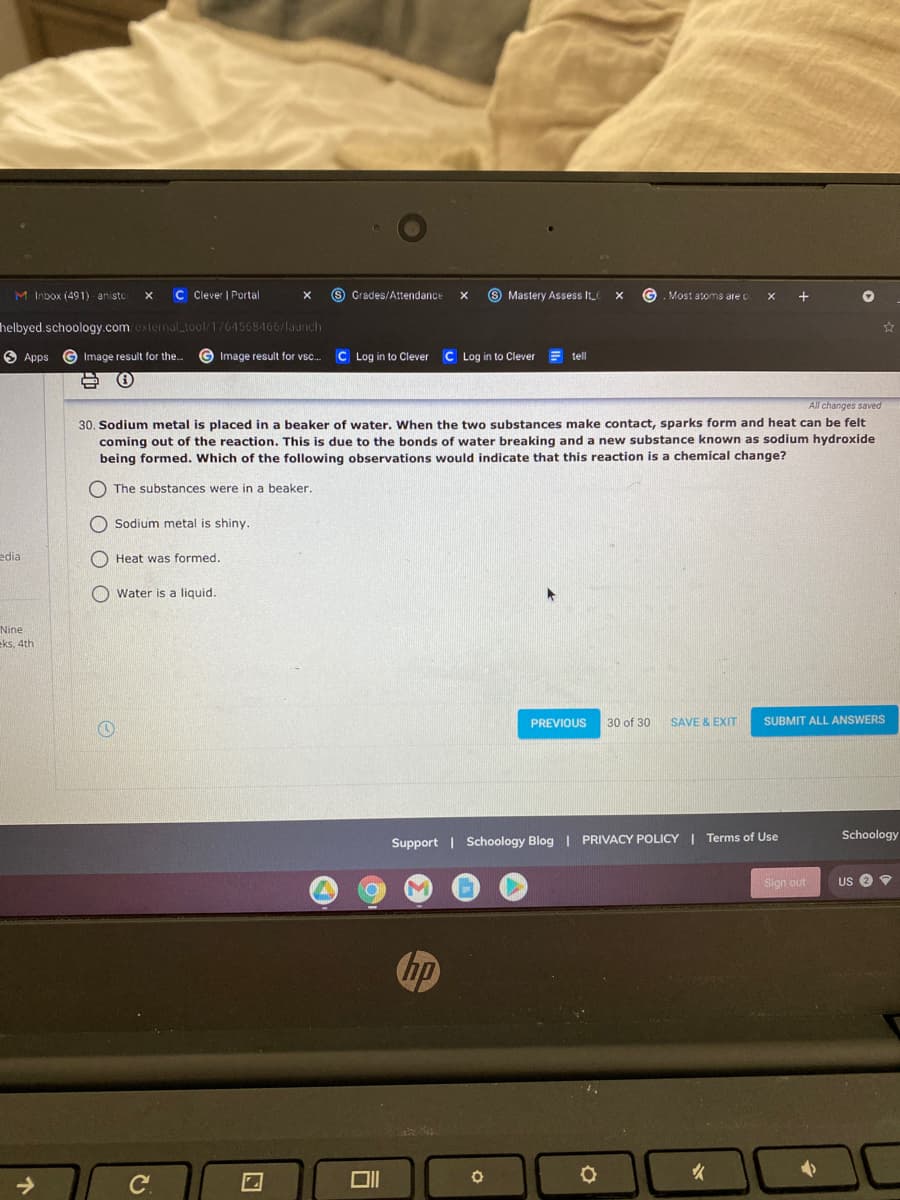
All changes saved 30. Sodium metal is placed in a beaker of water. When the two substances make contact, sparks form and heat can be felt coming out of the reaction. This is due to the bonds of water breaking and a new substance known as sodium hydroxide being formed. Which of the following observations would indicate that this reaction is a chemical change? O The substances were in a beaker. Sodium metal is shiny. O Heat was formed. Water is a liquid.
All changes saved 30. Sodium metal is placed in a beaker of water. When the two substances make contact, sparks form and heat can be felt coming out of the reaction. This is due to the bonds of water breaking and a new substance known as sodium hydroxide being formed. Which of the following observations would indicate that this reaction is a chemical change? O The substances were in a beaker. Sodium metal is shiny. O Heat was formed. Water is a liquid.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter2: Chemical Formulas, Equations, And Reaction Yields
Section: Chapter Questions
Problem 18P: A gaseous binary compound has a vapor density that is 2.53 times that of nitrogen at 100°C and...
Related questions
Question
Answer
Transcribed Image Text:M Inbox (491) anistc
C Clever | Portal
9 Grades/Attendance
O Mastery Assess It
Most atoms are c
helbyed.schoology.com external_tool/1764568466/launch
O Apps
G Image result for the..
© Image result for vsc.
C Log in to Clever
C Log in to Clever
E tell
All changes saved
30. Sodium metal is placed in a beaker of water. When the two substances make contact, sparks form and heat can be felt
coming out of the reaction. This is due to the bonds of water breaking and a new substance known as sodium hydroxide
being formed. Which of the following observations would indicate that this reaction is a chemical change?
O The substances were in a beaker.
O Sodium metal is shiny.
edia
O Heat was formed.
O water is a liquid.
Nine
eks, 4th
PREVIOUS
30 of 30
SAVE & EXIT
SUBMIT ALL ANSWERS
Schoology
Support | Schoology Blog | PRIVACY POLICY I Terms of Use
Sign out
Us O 9
hp
->
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning