aly Combined. Good examples of a solid mixture would be a salad or a bowl of trail mix. Solutions are mixtures too. A spoon full of sugar dissolved in water is an example of both a mixture and a solution. Because mixtures are physical combinations, they are easily separated. Here is a list of mixtures. Match the best method to separate the parts of each mixture. Boil the water. Collect the solid. ITEM BANK: Move to Top drag and drop answer here Gravel and rocks Dissolve salt in water. Pour through filter paper and collect the sand. drag and drop answer here Sand and gravel Remove iron filings with magnet. drag and drop answer here Sand and iron filings Sand and salt drag and drop answer here Use a screen to sift and separate the big pieces from smaller pieces. Sand and wood shavings drag and drop answer here Sugar water Pick out the bigger particles. drag and drop answer here Add water and float one type of particle.
aly Combined. Good examples of a solid mixture would be a salad or a bowl of trail mix. Solutions are mixtures too. A spoon full of sugar dissolved in water is an example of both a mixture and a solution. Because mixtures are physical combinations, they are easily separated. Here is a list of mixtures. Match the best method to separate the parts of each mixture. Boil the water. Collect the solid. ITEM BANK: Move to Top drag and drop answer here Gravel and rocks Dissolve salt in water. Pour through filter paper and collect the sand. drag and drop answer here Sand and gravel Remove iron filings with magnet. drag and drop answer here Sand and iron filings Sand and salt drag and drop answer here Use a screen to sift and separate the big pieces from smaller pieces. Sand and wood shavings drag and drop answer here Sugar water Pick out the bigger particles. drag and drop answer here Add water and float one type of particle.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter1: The Nature Of Chemistry
Section: Chapter Questions
Problem 105QRT
Related questions
Question
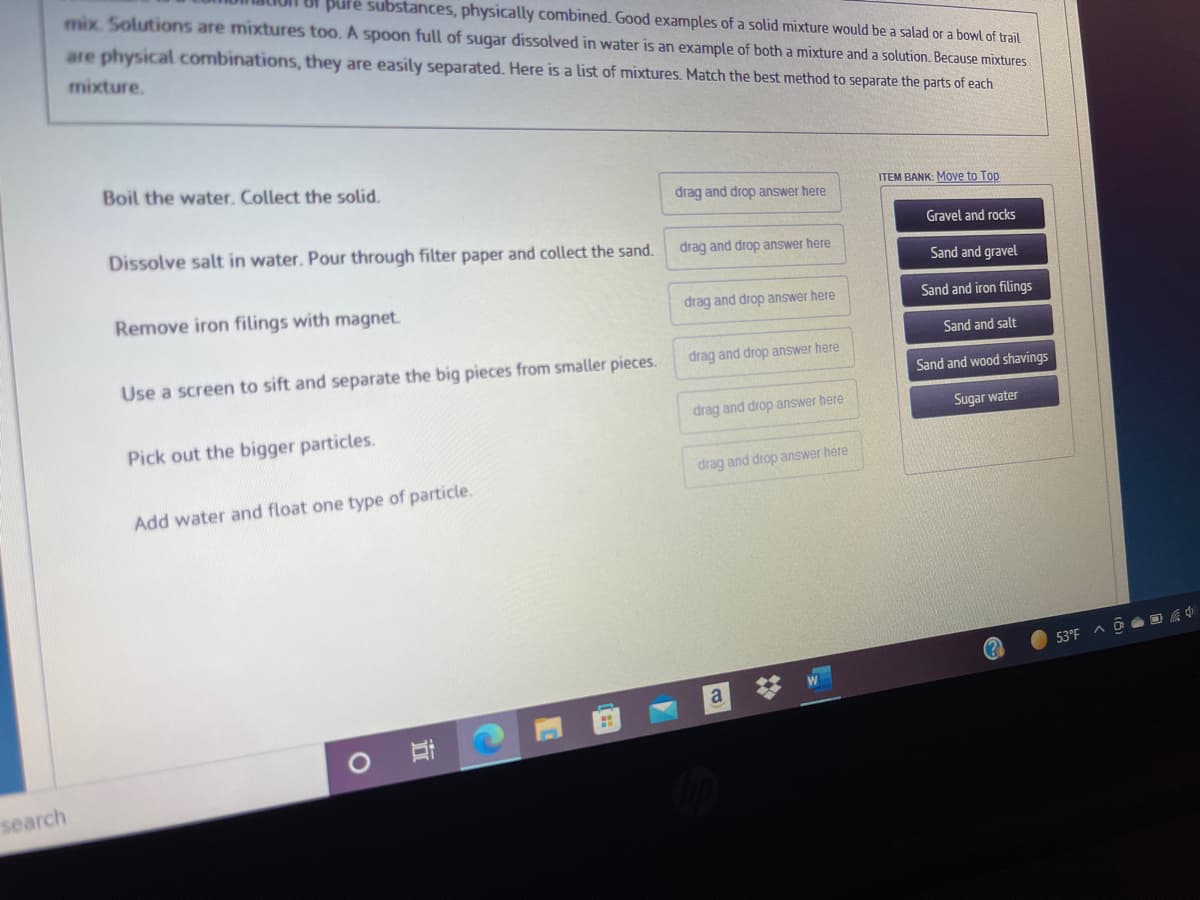
Transcribed Image Text:Bl pure substances, physically combined. Good examples of a solid mixture would be a salad or a bowl of trail
mix. Solutions are mixtures too. A spoon full of sugar dissolved in water is an example of both a mixture and a solution. Because mixtures
are physical combinations, they are easily separated. Here is a list of mixtures. Match the best method to separate the parts of each
mixture.
Boil the water. Collect the solid.
ITEM BANK: Move to Top
drag and drop answer here
Dissolve salt in water. Pour through filter paper and collect the sand.
Gravel and rocks
drag and drop answer here
Sand and gravel
Remove iron filings with magnet.
drag and drop answer here
Sand and iron filings
Sand and salt
Use a screen to sift and separate the big pieces from smaller pieces.
drag and drop answer here
Sand and wood shavings
drag and drop answer here
Sugar water
Pick out the bigger particles.
drag and drop answer here
Add water and float one type of particle.
53°F A O O
a
search
立
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co