An extraction experiment was carried out with the soxhlet extraction technique to obtain oil from a plant seed. In the experiment, 10 g of pla taken and extracted with 150 mL of hexane solvent. The oil-hexane mixture obtained at the end of the extraction will be separated in the rota and the mass and percent extraction efficiency of the collected oil will be calculated. The data obtained at the end of the experiment are give below. Accordingly, calculate the amount of oil obtained, the amount of oil obtained per 1 g of plant seed and the percent extraction efficiency b a mass conservation balance around the rotary evaporator. Weighing result (g) The tare of the sampled balloon 35 Initial mass (balloon + solvent + oil) 88.13 Tare the balloon in which the solvent was collected 45 93.47 At the end of the experiment, the balloon + solvent mass in which the solvent is collected
An extraction experiment was carried out with the soxhlet extraction technique to obtain oil from a plant seed. In the experiment, 10 g of pla taken and extracted with 150 mL of hexane solvent. The oil-hexane mixture obtained at the end of the extraction will be separated in the rota and the mass and percent extraction efficiency of the collected oil will be calculated. The data obtained at the end of the experiment are give below. Accordingly, calculate the amount of oil obtained, the amount of oil obtained per 1 g of plant seed and the percent extraction efficiency b a mass conservation balance around the rotary evaporator. Weighing result (g) The tare of the sampled balloon 35 Initial mass (balloon + solvent + oil) 88.13 Tare the balloon in which the solvent was collected 45 93.47 At the end of the experiment, the balloon + solvent mass in which the solvent is collected
Chapter14: Chromatography
Section: Chapter Questions
Problem 9P
Related questions
Question
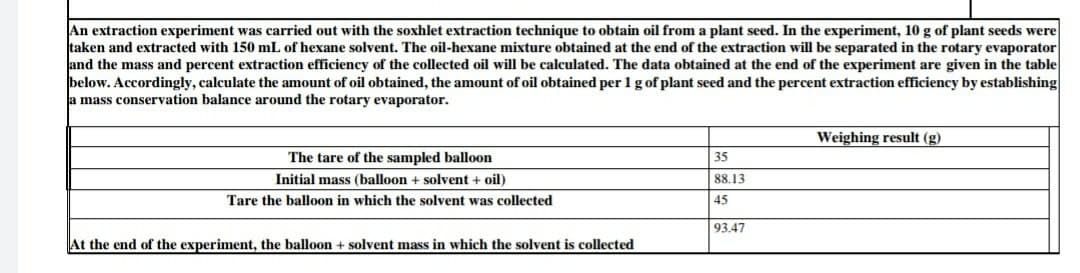
Transcribed Image Text:An extraction experiment was carried out with the soxhlet extraction technique to obtain oil from a plant seed. In the experiment, 10 g of plant seeds were
taken and extracted with 150 mL of hexane solvent. The oil-hexane mixture obtained at the end of the extraction will be separated in the rotary evaporator
and the mass and percent extraction efficiency of the collected oil will be calculated. The data obtained at the end of the experiment are given in the table
below. Accordingly, calculate the amount of oil obtained, the amount of oil obtained per 1 gof plant seed and the percent extraction efficiency by establishing
la mass conservation balance around the rotary evaporator.
Weighing result (g)
The tare of the sampled balloon
35
Initial mass (balloon + solvent + oil)
88.13
Tare the balloon in which the solvent was collected
45
93.47
At the end of the experiment, the balloon + solvent mass in which the solvent is collected
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

