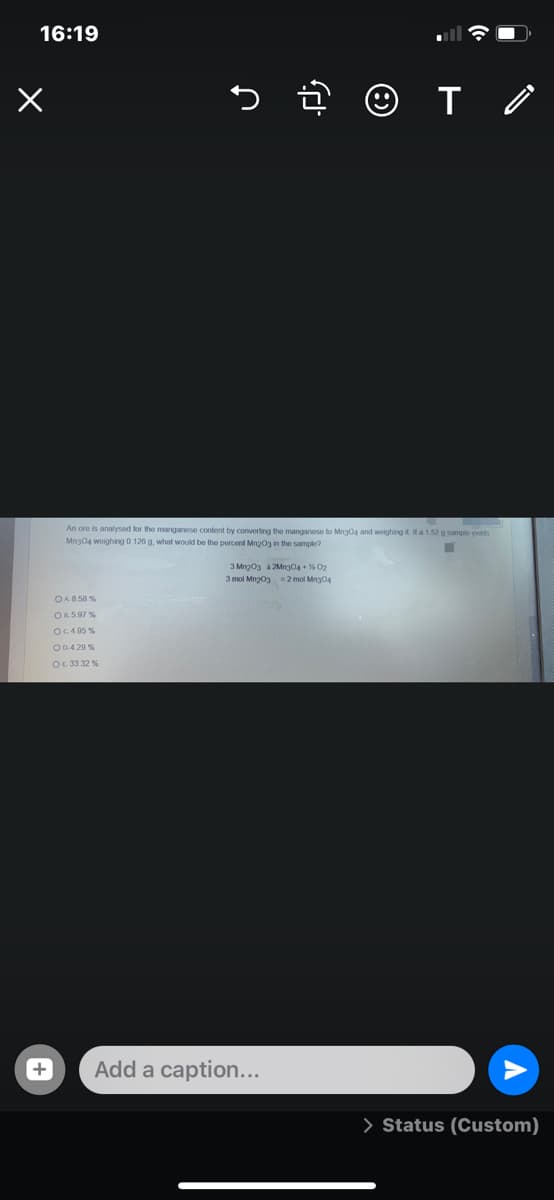
An ore is analysed lor the manganese content by converting the mangnese to Mo and woghing a152g sampe y Mrgoa woghng O 126 g what would be the percent Mgo3 in the sample?
An ore is analysed lor the manganese content by converting the mangnese to Mo and woghing a152g sampe y Mrgoa woghng O 126 g what would be the percent Mgo3 in the sample?
Chapter17: Complexation And Precipitation Reactions And Titrations
Section: Chapter Questions
Problem 17.23QAP
Related questions
Question
Transcribed Image Text:16:19
An ore is analyseod for the manganese content by converting the manganese to Mng04 and woighing it a 152 g sample yelds
Mng04 woighing 0.126 g, what would be the percent Mngog in the sample?
3 Mng03 a 2Mng04 + % 02
3 mol Mng03 =2 mol Mng04
OA858 %
OR5.97 %
OC4 95 %
O0.4 20 %
OE 33.32 %
Add a caption...
> Status (Custom)
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning