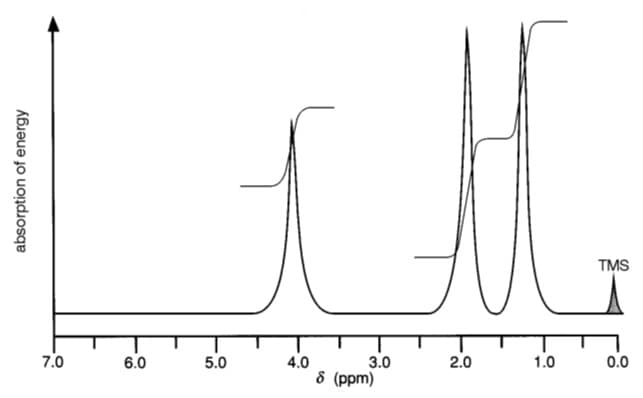
Analysis of a sweet-smelling, neutral compound of carbon, hydrogen and oxygen produced the following results: %C = 54.5; %H = 9.1. From its mass spectrum, the molecular ion had a mass/charge ratio of 88. Its infra-red spectrum showed a prominent peak at 1735 cm–1. Figure below shows the NMR spectrum of the compound. Which of the following is this compound? A. 3-hydroxybutanal B. Methyl propionate C. Ethyl acetate D. 2-methylpropanoic acid E. Methoxyacetone
Analysis of a sweet-smelling, neutral compound of carbon, hydrogen and oxygen produced the following results: %C = 54.5; %H = 9.1. From its mass spectrum, the molecular ion had a mass/charge ratio of 88. Its infra-red spectrum showed a prominent peak at 1735 cm–1. Figure below shows the NMR spectrum of the compound. Which of the following is this compound? A. 3-hydroxybutanal B. Methyl propionate C. Ethyl acetate D. 2-methylpropanoic acid E. Methoxyacetone
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter20: Kinetics
Section: Chapter Questions
Problem 20.80E
Related questions
Question
100%
Analysis of a sweet-smelling, neutral compound of carbon, hydrogen and oxygen produced the following results: %C = 54.5; %H = 9.1. From its mass spectrum, the molecular ion had a mass/charge ratio of 88. Its infra-red spectrum showed a prominent peak at 1735 cm–1. Figure below shows the NMR spectrum of the compound. Which of the following is this compound?
A. 3-hydroxybutanal
B. Methyl propionate
C. Ethyl acetate
D. 2-methylpropanoic acid
E. Methoxyacetone
Transcribed Image Text:TMS
3.0
2.0
1.0
0.0
4.0
8 (ppm)
7.0
6.0
5.0
absorption of energy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning