Aspirin can be synthesized in the lab by combining salicylic acid (C,H,O,) and acetic anhydride (C,H,O3) to form aspirin ( C,H,0,) and acetic acid (C,H,0,). The balanced equation for this reaction is C,H,0, + C,H,0, → C,H,O, +C,H,0, A student started with 3.41 ml. acetic anhydride (density = 1.08 g/ml.) and 2.61 g salicylic acid. The student synthesized 2.70 g of aspirin. Select the limiting reactant. salicylic acid (C,H,0,) O acetic acid (C, H,02) O acetic anhydride (C,H,0,) aspirin (C, H, 0,) Calculate the theoretical yield of aspirin (C,H,0,). theoretical yield: Calculate the percent yield for aspirin (C,H,0,). percent yield:
Aspirin can be synthesized in the lab by combining salicylic acid (C,H,O,) and acetic anhydride (C,H,O3) to form aspirin ( C,H,0,) and acetic acid (C,H,0,). The balanced equation for this reaction is C,H,0, + C,H,0, → C,H,O, +C,H,0, A student started with 3.41 ml. acetic anhydride (density = 1.08 g/ml.) and 2.61 g salicylic acid. The student synthesized 2.70 g of aspirin. Select the limiting reactant. salicylic acid (C,H,0,) O acetic acid (C, H,02) O acetic anhydride (C,H,0,) aspirin (C, H, 0,) Calculate the theoretical yield of aspirin (C,H,0,). theoretical yield: Calculate the percent yield for aspirin (C,H,0,). percent yield:
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 33QAP: A lead ore, galena, consisting mainly of lead(II) sulfide, is the principal source of lead. To...
Related questions
Question
Kindly refer to the attached picture
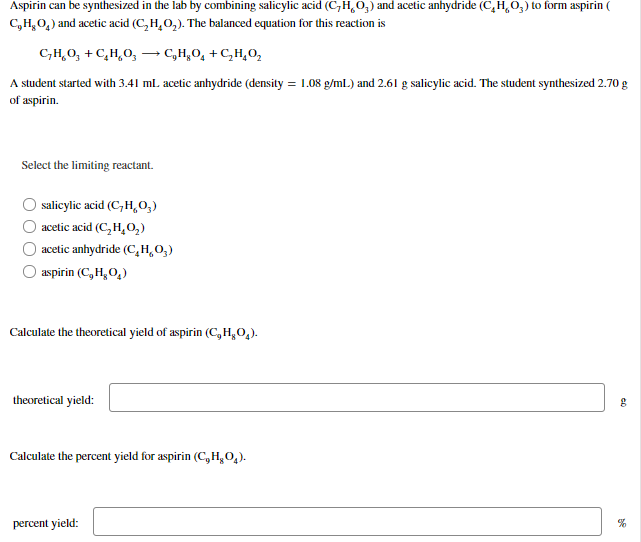
Transcribed Image Text:Aspirin can be synthesized in the lab by combining salicylic acid (C,H,O,) and acetic anhydride (C,H,O3) to form aspirin (
C,H,0,) and acetic acid (C,H,0,). The balanced equation for this reaction is
C,H,0, + C,H,0,
→ C,H,O, +C,H,0,
A student started with 3.41 ml. acetic anhydride (density = 1.08 g/ml.) and 2.61 g salicylic acid. The student synthesized 2.70 g
of aspirin.
Select the limiting reactant.
salicylic acid (C,H,0,)
O acetic acid (C, H,02)
O acetic anhydride (C,H,0,)
aspirin (C, H, 0,)
Calculate the theoretical yield of aspirin (C,H,0,).
theoretical yield:
Calculate the percent yield for aspirin (C,H,0,).
percent yield:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning