At 1200 the reduction of iron (III) oxide (Fe203) to elemental iron is nột spontan 2FE2O3(s) 4Fe(s) + 302(g) A;G° = +840 kJ the same temperature solid carbon spontaneously reacts with oxygen, to form C at by mixing carbon with Fe2O3 at this temperature the reduction can be made to p least 3 moles of carbon are present for every 2 moles of Fe2O3. (a) Write a balanced equation for the total reaction.
At 1200 the reduction of iron (III) oxide (Fe203) to elemental iron is nột spontan 2FE2O3(s) 4Fe(s) + 302(g) A;G° = +840 kJ the same temperature solid carbon spontaneously reacts with oxygen, to form C at by mixing carbon with Fe2O3 at this temperature the reduction can be made to p least 3 moles of carbon are present for every 2 moles of Fe2O3. (a) Write a balanced equation for the total reaction.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter18: Principles Of Chemical Reactivity: Entropy And Free Energy
Section: Chapter Questions
Problem 32PS
Related questions
Question
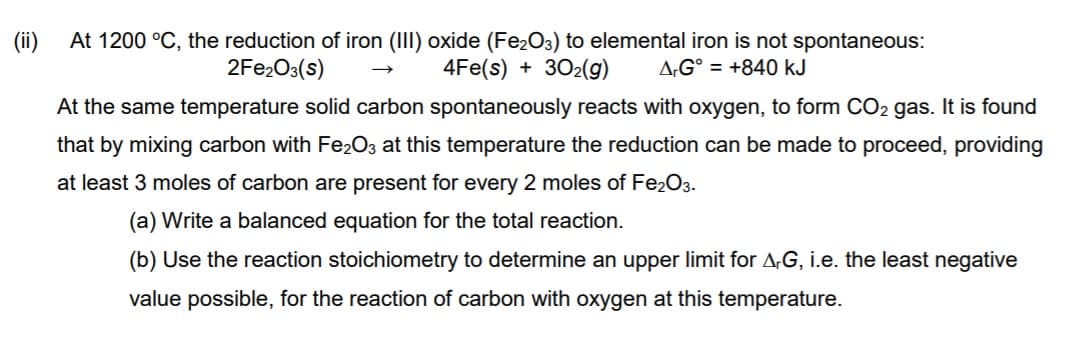
Transcribed Image Text:(ii)
At 1200 °C, the reduction of iron (III) oxide (Fe2O3) to elemental iron is not spontaneous:
4Fe(s) + 302(g)
2FE2O3(s)
A,G° = +840 kJ
At the same temperature solid carbon spontaneously reacts with oxygen, to form CO2 gas. It is found
that by mixing carbon with Fe2O3 at this temperature the reduction can be made to proceed, providing
at least 3 moles of carbon are present for every 2 moles of Fe2O3.
(a) Write a balanced equation for the total reaction.
(b) Use the reaction stoichiometry to determine an upper limit for A,G, i.e. the least negative
value possible, for the reaction of carbon with oxygen at this temperature.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning