Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.23PAE
Related questions
Question
Identify each compound below from its molecular formula and its 13C NMR spectrum.
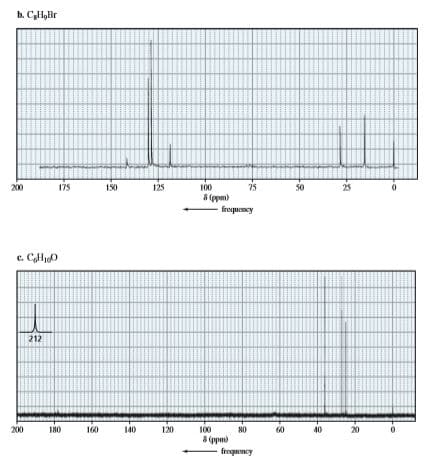
Transcribed Image Text:b. CyHyBr
200
175
150
125
100
75
50
25
8 (ppm)
frequemey
c. CHy0
212
200
180
160
120
100
20
8 (ppm)
frequency
Expert Solution
Step 1
The following rules should be followed to deduce the structure of unknown compound from the 13-C NMR data.
1) Check the number of signals in the spectrum which gives information regarding number of non-equivalent carbons in the molecule.
2) Identify the easily predictable signals in the spectrum, from which derive the possible fragments.
3) Subtract the chemical formula of all the derived fragments from the given molecular formula to know the remaining fragment.
4) At last, assemble all the fragments to get the structure of molecule which satisfies the given data.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning