Based on the table below, calculate F-factor and X in the water sample. (Berdasarkan jadual di bawah, kirakan faktor-F dan X dalam sampel air.) 7. Abs nitrite concentration Standard 0.032 0.4ppm Xppm Water sample 0.067
Based on the table below, calculate F-factor and X in the water sample. (Berdasarkan jadual di bawah, kirakan faktor-F dan X dalam sampel air.) 7. Abs nitrite concentration Standard 0.032 0.4ppm Xppm Water sample 0.067
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.31QE: A water solution of sodium hypochlorite (NaOCl) is used as laundry bleach. The concentration of...
Related questions
Question
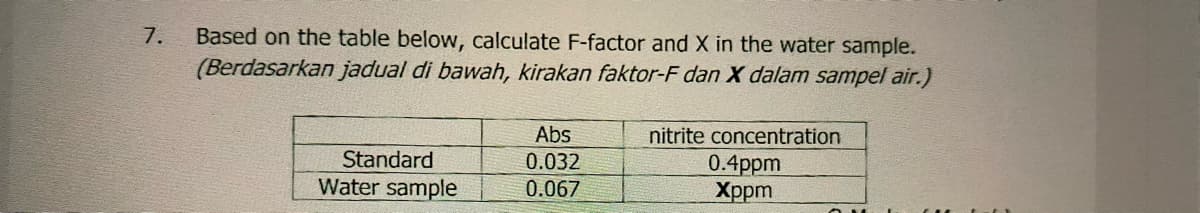
Transcribed Image Text:Based on the table below, calculate F-factor and X in the water sample.
(Berdasarkan jadual di bawah, kirakan faktor-F dan X dalam sampel air.)
7.
Abs
nitrite concentration
Standard
0.032
0.4ppm
Xppm
Water sample
0.067
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole