В. In his next experiment, he needs to determine the Ksp and identity of a sparingly soluble salt MF2. He was given two portions of 25.00 mL saturated MF2 solution. He used the first portion in the titration reaction against a 0.0110 M HNO3 standard solution. The table below shows the experimental data for this titration experiment. Initial buret reading 20.01 mL Final buret reading 29.51 mL Temperature 25.00° C 1. Determine the volume of HNO3 delivered during the titration. 2. Determine the number of moles of HNO, consumed in the reaction. 3. Write the balanced equation for the titration reaction. 4. Determine the molar solubility of the M2+ and F. 5. Determine the Ksp value of MF2. Report the final answer in proper SF.
В. In his next experiment, he needs to determine the Ksp and identity of a sparingly soluble salt MF2. He was given two portions of 25.00 mL saturated MF2 solution. He used the first portion in the titration reaction against a 0.0110 M HNO3 standard solution. The table below shows the experimental data for this titration experiment. Initial buret reading 20.01 mL Final buret reading 29.51 mL Temperature 25.00° C 1. Determine the volume of HNO3 delivered during the titration. 2. Determine the number of moles of HNO, consumed in the reaction. 3. Write the balanced equation for the titration reaction. 4. Determine the molar solubility of the M2+ and F. 5. Determine the Ksp value of MF2. Report the final answer in proper SF.
Chapter12: Spectrochemical Methods
Section: Chapter Questions
Problem 15P
Related questions
Question
4, 5
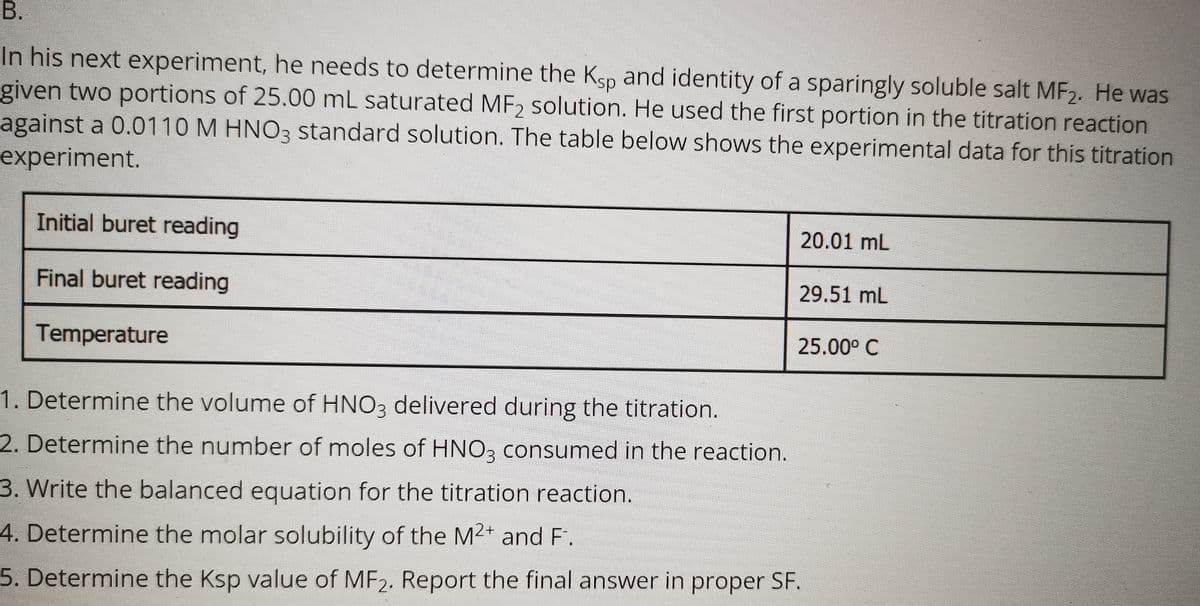
Transcribed Image Text:In his next experiment, he needs to determine the Ksp and identity of a sparingly soluble salt MF2. He was
given two portions of 25.00 mL saturated MF2 solution. He used the first portion in the titration reaction
against a 0.0110 M HNO3 standard solution. The table below shows the experimental data for this titration
experiment.
Initial buret reading
20.01 mL
Final buret reading
29.51 mL
Temperature
25.00° C
1. Determine the volume of HNO3 delivered during the titration.
2. Determine the number of moles of HNO3 consumed in the reaction.
3. Write the balanced equation for the titration reaction.
4. Determine the molar solubility of the M2+ and F.
5. Determine the Ksp value of MF2. Report the final answer in proper SF.
B.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax
