BASIC STOICHIOMETRY TO1/S11 X INCORRECT The answer is 78. Based on the balanced equation, Molar Mass (g/mol) 10Lİ + N2F4 - 4LİF + 2Lİ3N Li 6.941 calculate the formula units of LizN formed when 390 atoms of Li are reacted. N2F4 104.00 LIF 25.939 LizN Avogadro's No. 6.022x1023 mol1 34.830 [6.31E-22 exact number, no tolerance SHOW ANSWER Chem Help Question Attempts: 1 of 1 used
BASIC STOICHIOMETRY TO1/S11 X INCORRECT The answer is 78. Based on the balanced equation, Molar Mass (g/mol) 10Lİ + N2F4 - 4LİF + 2Lİ3N Li 6.941 calculate the formula units of LizN formed when 390 atoms of Li are reacted. N2F4 104.00 LIF 25.939 LizN Avogadro's No. 6.022x1023 mol1 34.830 [6.31E-22 exact number, no tolerance SHOW ANSWER Chem Help Question Attempts: 1 of 1 used
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter6: Chemical Calculations: Formula Masses, Moles, And Chemical Equations
Section: Chapter Questions
Problem 6.10EP: Select the quantity that contains the greater number of atoms in each of the following pairs of...
Related questions
Question
HELP me please
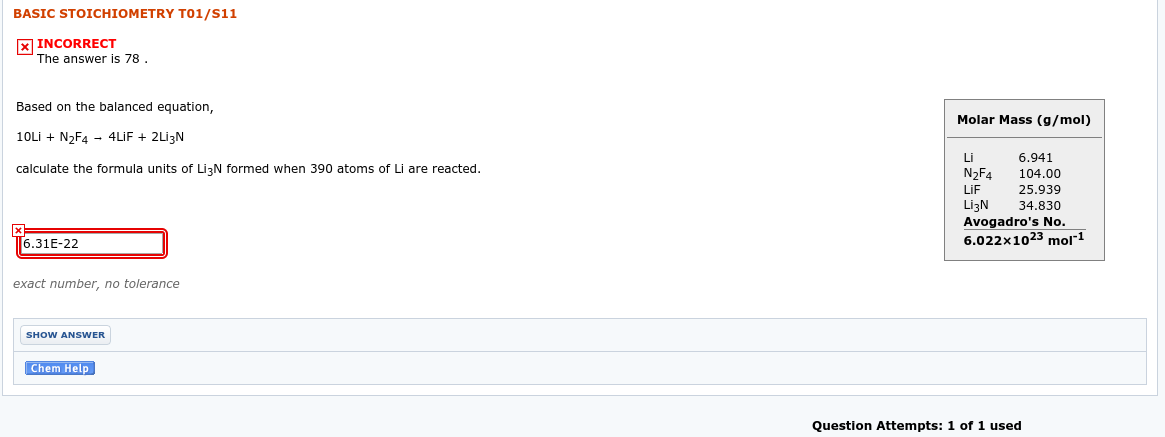
Transcribed Image Text:BASIC STOICHIOMETRY TO1/S11
X INCORRECT
The answer is 78.
Based on the balanced equation,
Molar Mass (g/mol)
10Lİ + N2F4 - 4LİF + 2Lİ3N
Li
6.941
calculate the formula units of LizN formed when 390 atoms of Li are reacted.
N2F4
104.00
LIF
25.939
LizN
Avogadro's No.
6.022x1023 mol1
34.830
[6.31E-22
exact number, no tolerance
SHOW ANSWER
Chem Help
Question Attempts: 1 of 1 used
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

