LIMITING REAGENTS TO2/S12 Based on the balanced equation Molar Mass (g/mol) 2C4Н10 + 1302 - 8СО2 + 10Н20 C4H10 calculate the number of excess reagent units remaining when 142 CaH10 molecules and 871 0, molecules react? 58.124 32.000 Co2 Н20 Avogadro's No. 6.022x1023 mol1 44.010 18.015 exact number, no tolerance Question Attempts: 0 of 1 used SUBMIT ANSWER SAVE FOR LATER
LIMITING REAGENTS TO2/S12 Based on the balanced equation Molar Mass (g/mol) 2C4Н10 + 1302 - 8СО2 + 10Н20 C4H10 calculate the number of excess reagent units remaining when 142 CaH10 molecules and 871 0, molecules react? 58.124 32.000 Co2 Н20 Avogadro's No. 6.022x1023 mol1 44.010 18.015 exact number, no tolerance Question Attempts: 0 of 1 used SUBMIT ANSWER SAVE FOR LATER
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 7ALQ
Related questions
Question
Please help
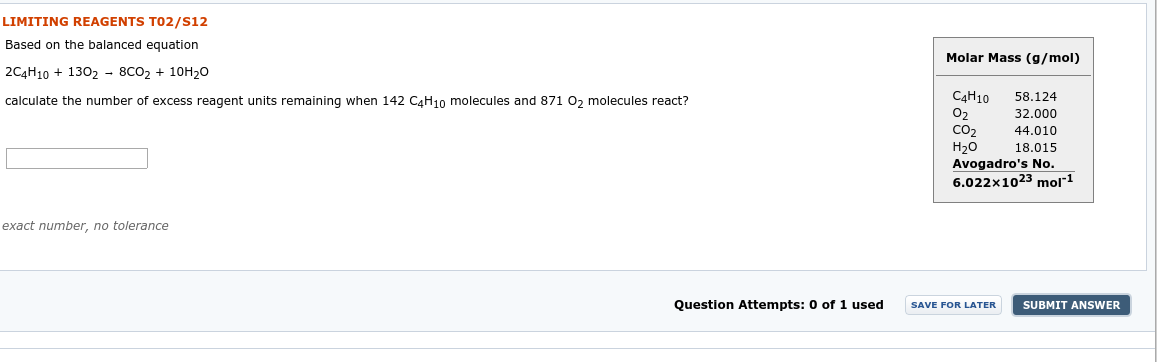
Transcribed Image Text:LIMITING REAGENTS TO2/S12
Based on the balanced equation
Molar Mass (g/mol)
2C4Н10 + 1302 - 8СО2 + 10Н20
C4H10
calculate the number of excess reagent units remaining when 142 CaH10 molecules and 871 0, molecules react?
58.124
32.000
Co2
Н20
Avogadro's No.
6.022x1023 mol1
44.010
18.015
exact number, no tolerance
Question Attempts: 0 of 1 used
SUBMIT ANSWER
SAVE FOR LATER
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning