On the right the Hill plot com- (b) pares the O2 binding properties of Hb Ya- kima with those of HbA in 0.1 M NaCl buff- Hb-Yakima ered to pH 7 with 0.01 M bis-Tris. Focus first on the line for "stripped Hb". This is the term for hemoglobin isolated from erythro- cytes with removal of all organic phosphate molecules that might bind to the protein in RBCS. You can see that 2,3-bisphospho- glycerate (BPG; labeled DPG according to old terminology) does not alter the O2 bind- ing affinity of Hb Yakima in contrast to HbA (although it was shown that BPG did bind to the deoxyHb Yakima molecule). Also, Hb Yakima is associated with markedly decreased allostery in the absence and presence of BPG, in comparison to HbA. IHP = inositol hexaphosphate, an artificial allosteric modifying ligand that binds more tightly than BPG. stripped Hb Hb-A •DPG +DPG n= 1.0 n = 2.3 n=2,5 +THP +IHP 0.5 0.5 1 5 10 50 po, ( mm Hg ) %3D On the right is a diagram copied from the lecture handout "Hemoglobin and Allo- stery" illustrating the environment of the side chain of BASP99 in T and R states. Explain which allosteric conformation of the Hb Ya- Asn G4 (102) Asp G1 (94) Thr C3 38 Thr C3 38 B2 oxygenation His FG4 97 Asp G1 (99) kima protein is destabilized and how this leads to the loss of allostery. Also, decide why the mutant BHis99 residue results in perturbation of the R to T allosteric equilib- rium, i.e., steric clashes or absence of H- bonding. Assume that the protein under- goes tertiary structural changes upon binding O2, as is most probable, what does the loss of allostery imply about the O2 binding affinity of the protein in each tertiary conformational state? Thr C6 41 Thr C6 41 Tyr C7 (42) His FG4 97 Pro CD2 44 Pro CD2 44 fa) T State (deoxy) (b) R State (oxy)
On the right the Hill plot com- (b) pares the O2 binding properties of Hb Ya- kima with those of HbA in 0.1 M NaCl buff- Hb-Yakima ered to pH 7 with 0.01 M bis-Tris. Focus first on the line for "stripped Hb". This is the term for hemoglobin isolated from erythro- cytes with removal of all organic phosphate molecules that might bind to the protein in RBCS. You can see that 2,3-bisphospho- glycerate (BPG; labeled DPG according to old terminology) does not alter the O2 bind- ing affinity of Hb Yakima in contrast to HbA (although it was shown that BPG did bind to the deoxyHb Yakima molecule). Also, Hb Yakima is associated with markedly decreased allostery in the absence and presence of BPG, in comparison to HbA. IHP = inositol hexaphosphate, an artificial allosteric modifying ligand that binds more tightly than BPG. stripped Hb Hb-A •DPG +DPG n= 1.0 n = 2.3 n=2,5 +THP +IHP 0.5 0.5 1 5 10 50 po, ( mm Hg ) %3D On the right is a diagram copied from the lecture handout "Hemoglobin and Allo- stery" illustrating the environment of the side chain of BASP99 in T and R states. Explain which allosteric conformation of the Hb Ya- Asn G4 (102) Asp G1 (94) Thr C3 38 Thr C3 38 B2 oxygenation His FG4 97 Asp G1 (99) kima protein is destabilized and how this leads to the loss of allostery. Also, decide why the mutant BHis99 residue results in perturbation of the R to T allosteric equilib- rium, i.e., steric clashes or absence of H- bonding. Assume that the protein under- goes tertiary structural changes upon binding O2, as is most probable, what does the loss of allostery imply about the O2 binding affinity of the protein in each tertiary conformational state? Thr C6 41 Thr C6 41 Tyr C7 (42) His FG4 97 Pro CD2 44 Pro CD2 44 fa) T State (deoxy) (b) R State (oxy)
Biochemistry
6th Edition
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Reginald H. Garrett, Charles M. Grisham
Chapter18: Glycolysis
Section: Chapter Questions
Problem 25P: Using the ActiveModel for phosphofructokinase (Trypanosoma), describe the difference between the...
Related questions
Question
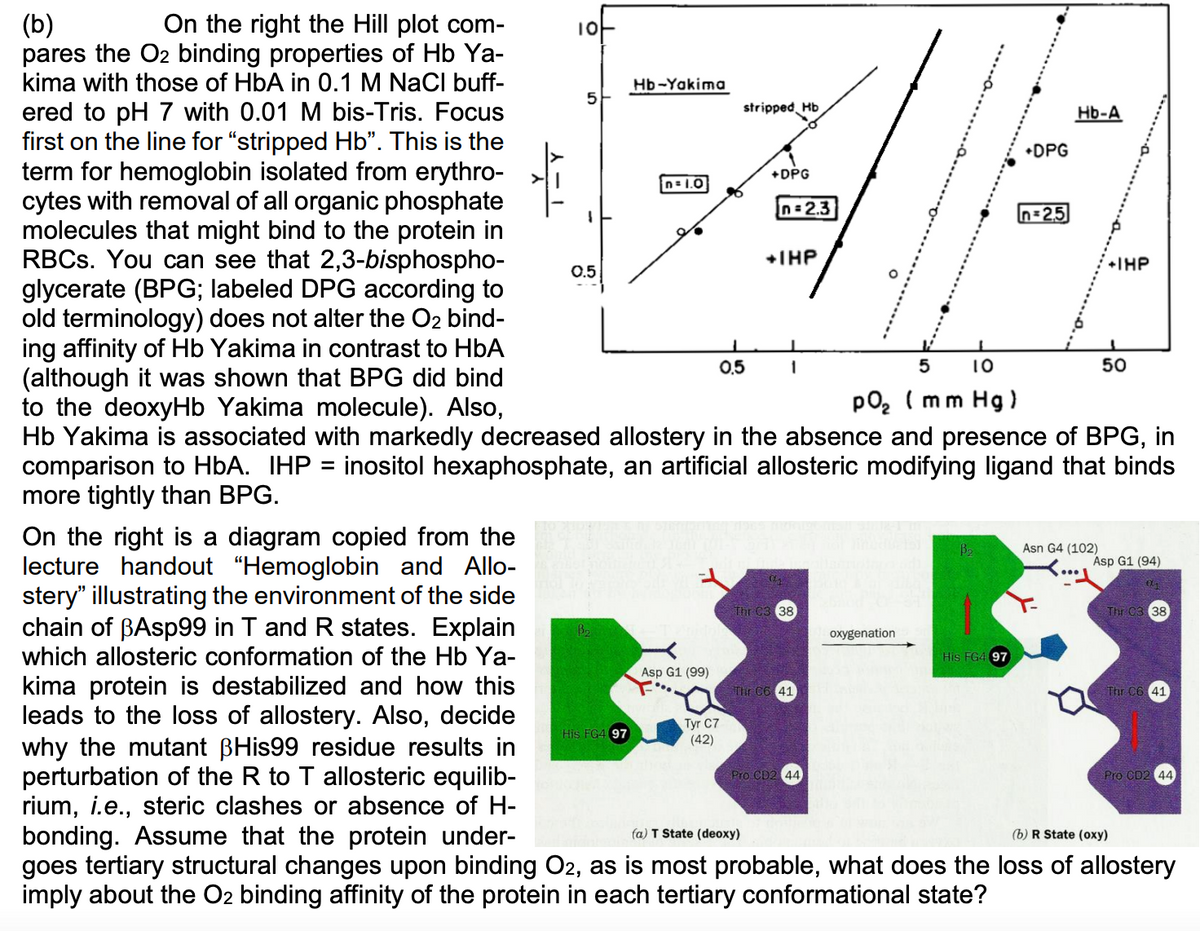
Transcribed Image Text:On the right the Hill plot com-
(b)
pares the O2 binding properties of Hb Ya-
kima with those of HbA in 0.1 M NaCl buff-
Hb-Yakima
ered to pH 7 with 0.01 M bis-Tris. Focus
first on the line for "stripped Hb". This is the
term for hemoglobin isolated from erythro-
cytes with removal of all organic phosphate
molecules that might bind to the protein in
RBCS. You can see that 2,3-bisphospho-
glycerate (BPG; labeled DPG according to
old terminology) does not alter the O2 bind-
ing affinity of Hb Yakima in contrast to HbA
(although it was shown that BPG did bind
to the deoxyHb Yakima molecule). Also,
Hb Yakima is associated with markedly decreased allostery in the absence and presence of BPG, in
comparison to HbA. IHP = inositol hexaphosphate, an artificial allosteric modifying ligand that binds
more tightly than BPG.
stripped Hb
Hb-A
•DPG
+DPG
n= 1.0
n = 2.3
n=2,5
+THP
+IHP
0.5
0.5
1
5
10
50
po, ( mm Hg )
%3D
On the right is a diagram copied from the
lecture handout "Hemoglobin and Allo-
stery" illustrating the environment of the side
chain of BASP99 in T and R states. Explain
which allosteric conformation of the Hb Ya-
Asn G4 (102)
Asp G1 (94)
Thr C3 38
Thr C3 38
B2
oxygenation
His FG4 97
Asp G1 (99)
kima protein is destabilized and how this
leads to the loss of allostery. Also, decide
why the mutant BHis99 residue results in
perturbation of the R to T allosteric equilib-
rium, i.e., steric clashes or absence of H-
bonding. Assume that the protein under-
goes tertiary structural changes upon binding O2, as is most probable, what does the loss of allostery
imply about the O2 binding affinity of the protein in each tertiary conformational state?
Thr C6 41
Thr C6 41
Tyr C7
(42)
His FG4 97
Pro CD2 44
Pro CD2 44
fa) T State (deoxy)
(b) R State (oxy)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning