Calculate the standard free-energy change, deltaG'o, for the reaction in which acetaldehyde is reduced by the biological electron carrier NADH in the reaction acetaldehyde + NADH + H+ → ethanol + NAD+. Then calculate the actual free-energy change, deltaG, when the acetaldehyde is 1.51 M, the NADH is 1.02 M, the ethanol is 0.13 M and the NAD+ is 0.19 M, at 33.35oC and pH = 7. Give the actual free-energy change in kJ/mol to one decimal place. See Table 13-7b for the E'o values.
Calculate the standard free-energy change, deltaG'o, for the reaction in which acetaldehyde is reduced by the biological electron carrier NADH in the reaction acetaldehyde + NADH + H+ → ethanol + NAD+. Then calculate the actual free-energy change, deltaG, when the acetaldehyde is 1.51 M, the NADH is 1.02 M, the ethanol is 0.13 M and the NAD+ is 0.19 M, at 33.35oC and pH = 7. Give the actual free-energy change in kJ/mol to one decimal place. See Table 13-7b for the E'o values.
Biochemistry
6th Edition
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Reginald H. Garrett, Charles M. Grisham
Chapter21: Photosynthesis
Section: Chapter Questions
Problem 13P
Related questions
Question
Calculate the standard free-energy change, deltaG'o, for the reaction in which acetaldehyde is reduced by the biological electron carrier NADH in the reaction acetaldehyde + NADH + H+ → ethanol + NAD+. Then calculate the actual free-energy change, deltaG, when the acetaldehyde is 1.51 M, the NADH is 1.02 M, the ethanol is 0.13 M and the NAD+ is 0.19 M, at 33.35oC and pH = 7. Give the actual free-energy change in kJ/mol to one decimal place. See Table 13-7b for the E'o values.
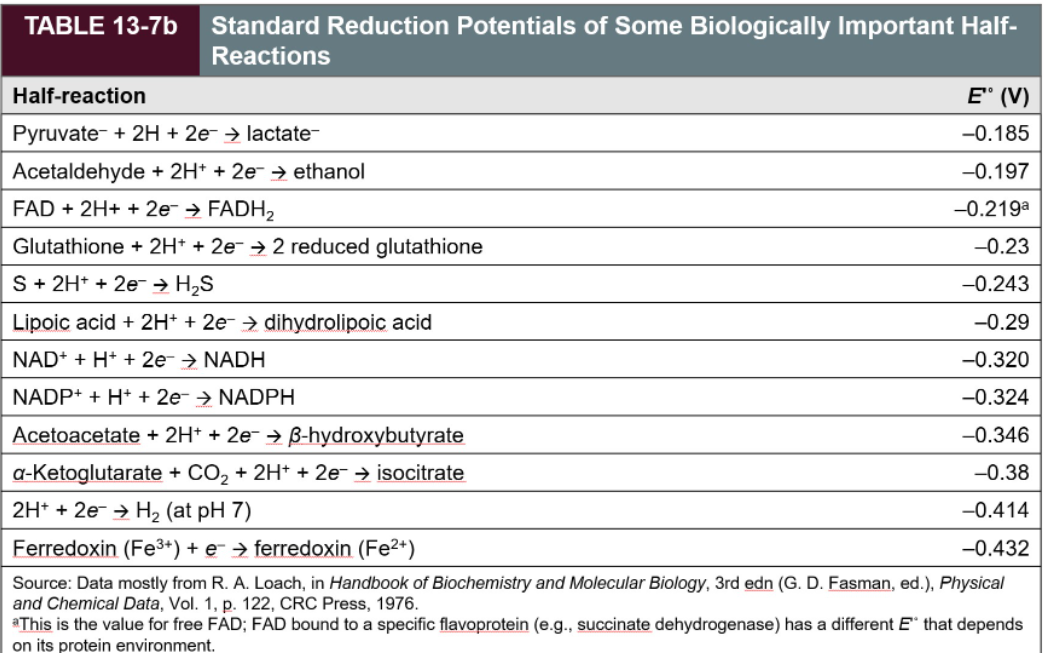
Transcribed Image Text:TABLE 13-7b
Standard Reduction Potentials of Some Biologically Important Half-
Reactions
Half-reaction
E" (V)
Pyruvate- + 2H + 2e- → lactate-
-0.185
Acetaldehyde + 2H* + 2e- → ethanol
-0.197
FAD + 2H+ + 2e- → FADH2
-0.219a
Glutathione + 2H* + 2e- → 2 reduced glutathione
-0.23
S+ 2H* + 2e¯ → H,S
-0.243
Lipoic acid + 2H* + 2e- → dihydrolipoic acid
-0.29
NAD+ + H+ + 2e- → NADH
-0.320
NADP+ + H*+ + 2e- → NADPH
-0.324
Acetoacetate + 2H* + 2e- → B-hydroxybutyrate
-0.346
a-Ketoglutarate + CO2 + 2H* + 2e- → isocitrate
2H* + 2e → H2 (at pH 7)
-0.38
-0.414
Ferredoxin (Fe3+) + e¯ → ferredoxin (Fe2*)
-0.432
Source: Data mostly from R. A. Loach, in Handbook of Biochemistry and Molecular Biology, 3rd edn (G. D. Fasman, ed.), Physical
and Chemical Data, Vol. 1, p. 122, CRC Press, 1976.
*This is the value for free FAD; FAD bound to a specific flavoprotein (e.g., succinate dehydrogenase) has a different E" that depends
on its protein environment.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning