C The Average Adult Human Burr x b Search results for 'Convert 3.2 x app.101edu.co mail YouTube Maps 1o Chem101 Update 国 Reading Question 3 of 23 Sub The average adult human burns 2.00 x 10 kcal per day in energy. What is this rate in kJ per hour ? MOUNT ADD FACTOR ANSWER RESET *( ) 60 4184 2.01 x 106 4.184 1.15 x 105 1 24 0.001 20.0 349 2.00 x 10 1000 kcal/day days kJ cal kJ/hr hr min kcal
C The Average Adult Human Burr x b Search results for 'Convert 3.2 x app.101edu.co mail YouTube Maps 1o Chem101 Update 国 Reading Question 3 of 23 Sub The average adult human burns 2.00 x 10 kcal per day in energy. What is this rate in kJ per hour ? MOUNT ADD FACTOR ANSWER RESET *( ) 60 4184 2.01 x 106 4.184 1.15 x 105 1 24 0.001 20.0 349 2.00 x 10 1000 kcal/day days kJ cal kJ/hr hr min kcal
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 75QAP: Some solar-heated homes use large beds of rocks to store heat. (a) How much heat is absorbed by...
Related questions
Question
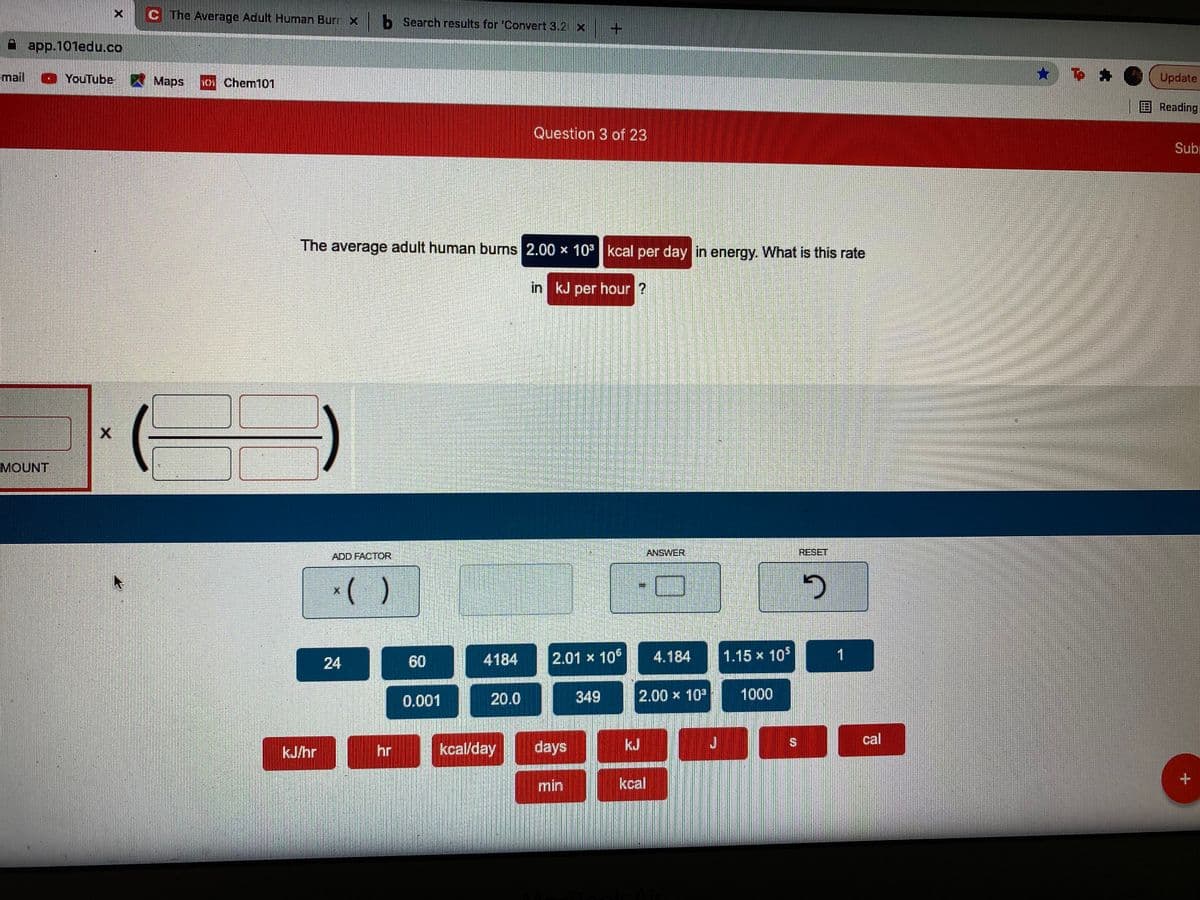
Transcribed Image Text:C The Average Adult Human Burr x b Search results for 'Convert 3.2 x
A app.101edu.co
Email YouTube
A Maps
101 Chem101
Update
EReading
Question 3 of 23
Subr
The average adult human burns 2.00 x 10 kcal per day in energy. What is this rate
in kJ per hour ?
MOUNT
ADD FACTOR
ANSWER
RESET
24
60
4184
2.01 x 106
4.184
1.15 x 10$
1
0.001
20.0
349
2.00 x 10
1000
kcal/day
days
kJ
cal
kJ/hr
hr
min
kcal
%24
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning