Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 93AP
Related questions
Question
Transcribed Image Text:tural Sciences
Chemistry And Polymer Science / Chemie - Chemistry-144/ Praktika | Practicals
es 3 Verslag
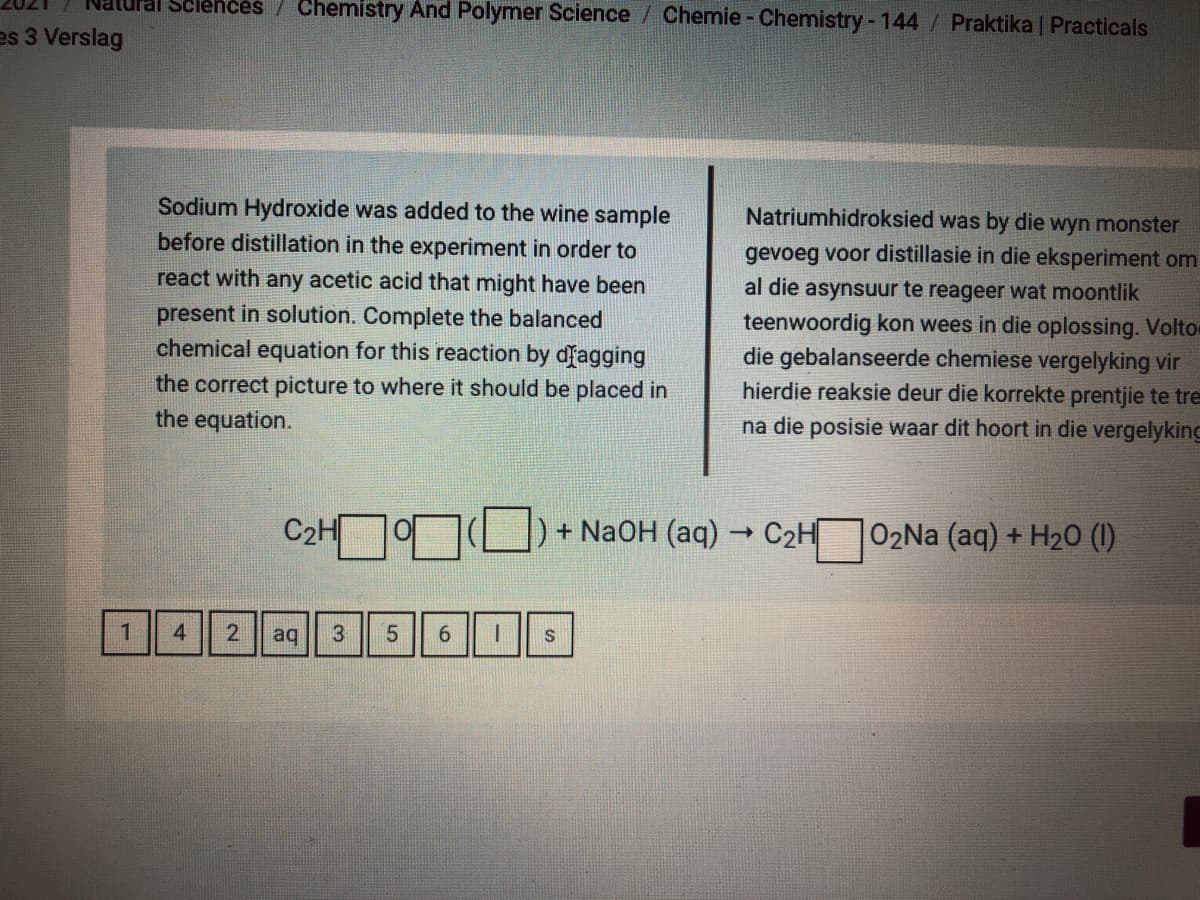
Sodium Hydroxide was added to the wine sample
before distillation in the experiment in order to
Natriumhidroksied was by die wyn monster
gevoeg voor distillasie in die eksperiment om
al die asynsuur te reageer wat moontlik
teenwoordig kon wees in die oplossing. Voltor
die gebalanseerde chemiese vergelyking vir
hierdie reaksie deur die korrekte prentjie te tre
na die posisie waar dit hoort in die vergelyking
react with any acetic acid that might have been
present in solution. Complete the balanced
chemical equation for this reaction by dfagging
the correct picture to where it should be placed in
the equation.
C2H
+ NaOH (aq) -
C2H
|02Na (aq) + H20 (1)
1.
4
aq
6.
S
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning