Cal Culate the amount of lime(-Ca0) that Gnbe grepared by heating 200 kgolimestone that is 95% pure Cacos M.wt. CaCog=l00 M.Wt Cao-56-1 Ca Cos Cao + Co
Cal Culate the amount of lime(-Ca0) that Gnbe grepared by heating 200 kgolimestone that is 95% pure Cacos M.wt. CaCog=l00 M.Wt Cao-56-1 Ca Cos Cao + Co
Chapter4: Organic Compounds: Cycloalkanes And Their Stereochemistry
Section4.SE: Something Extra
Problem 52AP: Using molecular models as well as structural drawings, explain why trans-decalin is rigid and cannot...
Related questions
Question
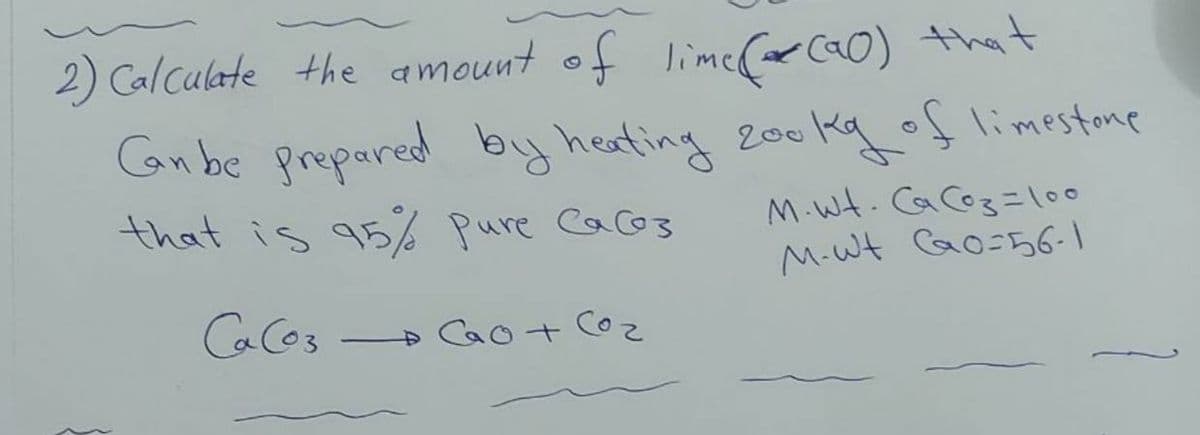
Transcribed Image Text:2) Calculate the amount of lime(r ca0) that
Gan be prepared by heafing 200kg of limestone
that is 95% pure Cacos
M.Wt. CaCoz=l00
M-W Go=56-1
CaCos
Cao+ Co
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning