Calcium carbide reacts with water to produce acetylene gas according to the following equation: CaC2(s) + 2H20(1)C2H2(g) + Ca(OH)2(aq) The product gas, C2H2, is collected over water at a temperature of 20 °C and a pressure of 755 mm Hg. If the wet C,H2 gas formed occupies a volume of 9.76 L, the number of moles of CaC2 reacted was mol. The vapor pressure of water is 17.5 mm Hg at 20 °C.
Calcium carbide reacts with water to produce acetylene gas according to the following equation: CaC2(s) + 2H20(1)C2H2(g) + Ca(OH)2(aq) The product gas, C2H2, is collected over water at a temperature of 20 °C and a pressure of 755 mm Hg. If the wet C,H2 gas formed occupies a volume of 9.76 L, the number of moles of CaC2 reacted was mol. The vapor pressure of water is 17.5 mm Hg at 20 °C.
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter5: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 5.114P: 5-114 Carbon dioxide gas, saturated with water vapor, can be produced by the addition of aqueous...
Related questions
Question
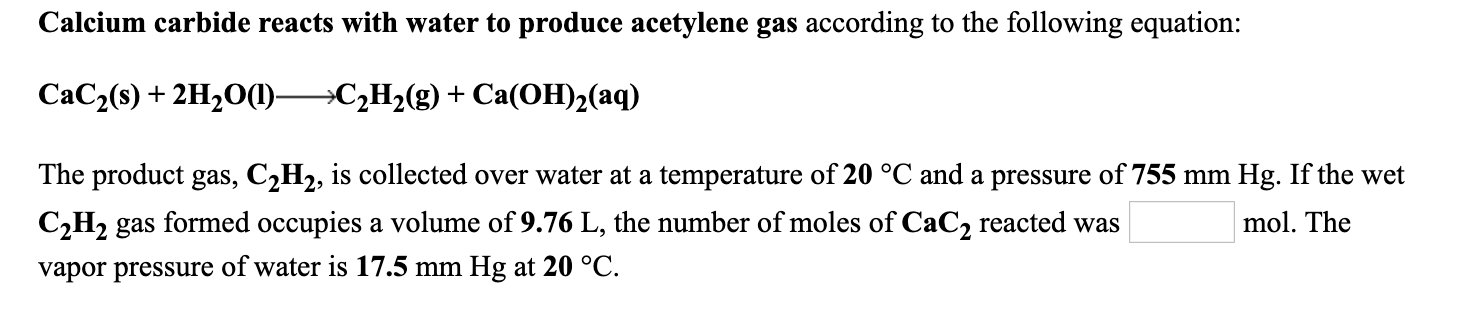
Transcribed Image Text:Calcium carbide reacts with water to produce acetylene gas according to the following equation:
CaC2(s) + 2H20(1)C2H2(g) + Ca(OH)2(aq)
The product gas, C2H2, is collected over water at a temperature of 20 °C and a pressure of 755 mm Hg. If the wet
C,H2 gas formed occupies a volume of 9.76 L, the number of moles of CaC2 reacted was
mol. The
vapor pressure of water is 17.5 mm Hg at 20 °C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 1 images

Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
