
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Resubmitting this question because I got denied the first time. I'm not giving you the values of standard S from appendix C because it's NOT RIGHT. My teacher flat out told me that the values from the book are wrong so please don't deny this question again because I didn't supply the book values.
Can someone please solve this question I keep getting it wrong I got -73.3J/K*mol but it says its wrong. Can someone help me get the right answer.
Thank you
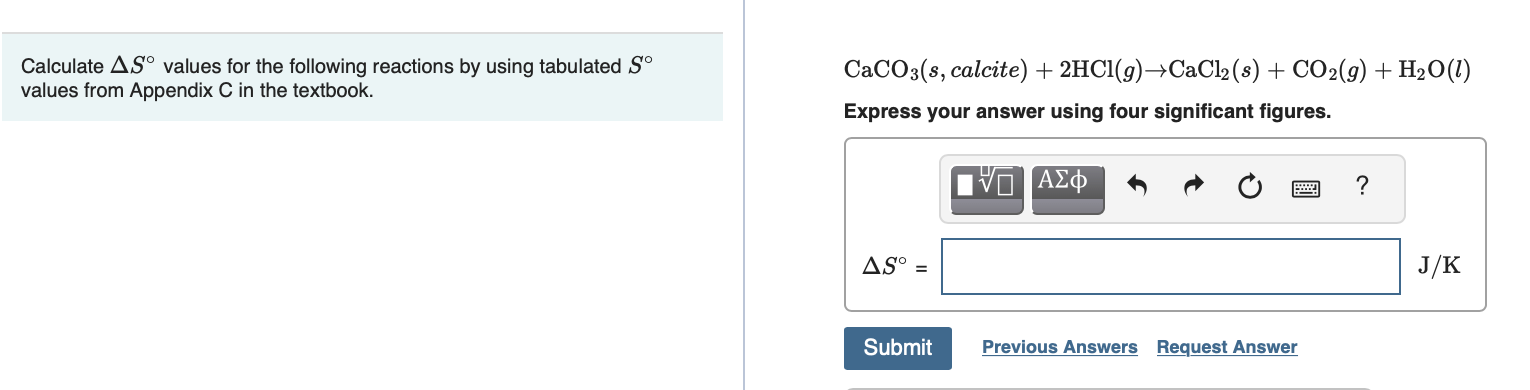
Transcribed Image Text:Calculate AS° values for the following reactions by using tabulated S°
values from Appendix C in the textbook.
CaCO3(s, calcite) + 2HC1(9)→CaCl2 (8) + CO2(9) + H20(1)
Express your answer using four significant figures.
AS° =
J/K
Submit
Previous Answers Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1.What happens to the heat capacity (CP) of a metal sample if its mass is doubled? Explain your answer[hint: examine the units and consider using the terms “intensive” or “extensive” in your answer]. 2.What happens to the specific heat (cs) of a metal sample if its mass is doubled? Explain your answer [hint:examine the units and consider using the terms “intensive” or “extensive” in your answer].arrow_forward4. The reaction of 250.0 mL of a 1.00 M hydrochloric acid solution with 250.0 mL of a 1.00 M sodium hydroxide solution was carried out in a constant pressure calorimeter. The total heat capacity of the calorimeter plus solutions was 6.45 kJ/K. The temperature of the calorimeter and solutions increased by 2.11°C. What is AH (in kJ) for the neutralization of 1.00 mol HCl(aq) by NaOH(aq)? A) -54.4 B) -21.2 +12.6 +54.4 E) -12.6arrow_forwardConsider an ideal gas enclosed in a 1.00 L container at an internal pressure of 24.0 atm. Calculate the work, w, if the gas expands against a constant external pressure of 1.00 atm to a final volume of 24.0 L. -23.0 W = Now calculate the work done if this process is carried out in two steps. 1. First, let the gas expand against a constant external pressure of 1.50 atm to a volume of 16.0 L. 2. From the end point of step 1, let the gas expand to 24.0 L against a constant external pressure of 1.00 atm. W = J Jarrow_forward
- Copper has been used for thousands of years, either as a pure metal or in alloys. It is frequently used today in the production of wires and cables. Copper can be obtained through smelting or recycling. Determine the energy associated with each of these processes in order to recycle 1.40 mol Cu. The smelting of copper occurs by the balanced chemical equation: CuO(s) +CO(g) → Cu(s) +CO,(g) where AHtCuo is = - 155 kJ/mol. Assume the process of recycling copper is simplified to just the melting of the solid Cu starting at 25°C. The melting point of Cu is 1084.5°C with AH®fus = 13.0 kJ/mol and a molar heat capacity, CPCU = 24.5 J/mol:°C.arrow_forwardA gas is compressed from an initial volume of 5.70 L to a final volume of 1.24 L by an external pressure of 1.00 atm. During the compression the gas releases 125 J of heat. What is the change in internal energy of the gas?arrow_forwardMISSED THIS? Read Section 7.6 (Pages 281- 284), Watch IWE 7.7 View Available Hint(s) Nitromethane (CH,NO2) burns in air to produce significant amounts of heat via the following reaction Hint 1. Calculate the number of moles of nitromethane 2CH,NO, (1) +O2(g)→ 2CO2(g) +3H,O(1) + N2(g) The value of AH is related to the balanced chemical equation by the number of moles of nitromethane. Calculate the number of moles of nitromethane in a 2.73-kg sample. AHn -1418 kJ Express your answer in moles. mol CH, NO2 44.72 Submit Previous Answers Request Answer X Incorrect; Try Again; 3 attempts remaining Convert the mass of nitromethane from kg to g. Then use the molar mass of nitromethane as a conversion factor to obtain a value in mol HA Value Units = barrow_forward
- 10 A student heats 84.17 mL of water to 95.27°C using a hot plate. The heated water is added to a calorimeter containing 73.92 mL of cold water. The water temperature in the calorimeter rises from 2.15°C to 37.48°C. The specific heat capacity of water is 4.184 J and the density of water is g. °C 1.00 mL Assuming that heat was transferred from the hot water to the cold water and the calorimeter, determine the heat capacity of the calorimeter. J Heat capacity of calorimeter = °Carrow_forward(Incorrect) Consider the following reaction: 2 PbS (s) + 3 02 (g) → 2 PbO (s) +2 SO₂ (g) AHxn=-827.4 kJ What mass of PbS has reacted if 765 kJ of heat is produced? You answered: 221. The correct answer is: 442.0g Aarrow_forwardA 56.5 g sample of iron is put into a calorimeter (see sketch at right) that contains 300.0 g of water. The iron sample starts off at 86.7 °C and the temperature of the water starts off at 21.0 °C. When the temperature of the water stops changing it's 22.8 °C. The pressure remains constant at 1 atm. Calculate the specific heat capacity of iron according to this experiment. Be sure your answer is rounded to 2 significant digits. J 0₂-C x10 x thermometer insulated container water sample a calorimeterarrow_forward
- 4. Consider a 15.0 g piece of tin metal initially at 25 oC, What is its specific heat if adding 250 cal to it causes its temperature to increase to 125 oC.arrow_forwardA student is attempting to determine the heat capacity of a Styrofoam cup calorimeter by pouring hot water into a Styrofoam cup containing cold water. The student determined the mass of the cold water to be 21.2455 g and its initial temperature to be 20.36 °C. The mass of the hot water was 24.2646 g and its initial temperature as 34.54 °C. The final temperature of the water after mixing was determined to be 24.57°C. The specific heat capacity of the water is 4.184 J/(g•°C). What is the heat capacity of the Styrofoam cup calorimeter? Assume the temperature of the calorimeter is the same temperature as the cold water. 4.184 J/°C 132.5 J/°Carrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY