Chapter13: Dimensional Analysis/units Conversion
Section: Chapter Questions
Problem 2.4P
Related questions
Question
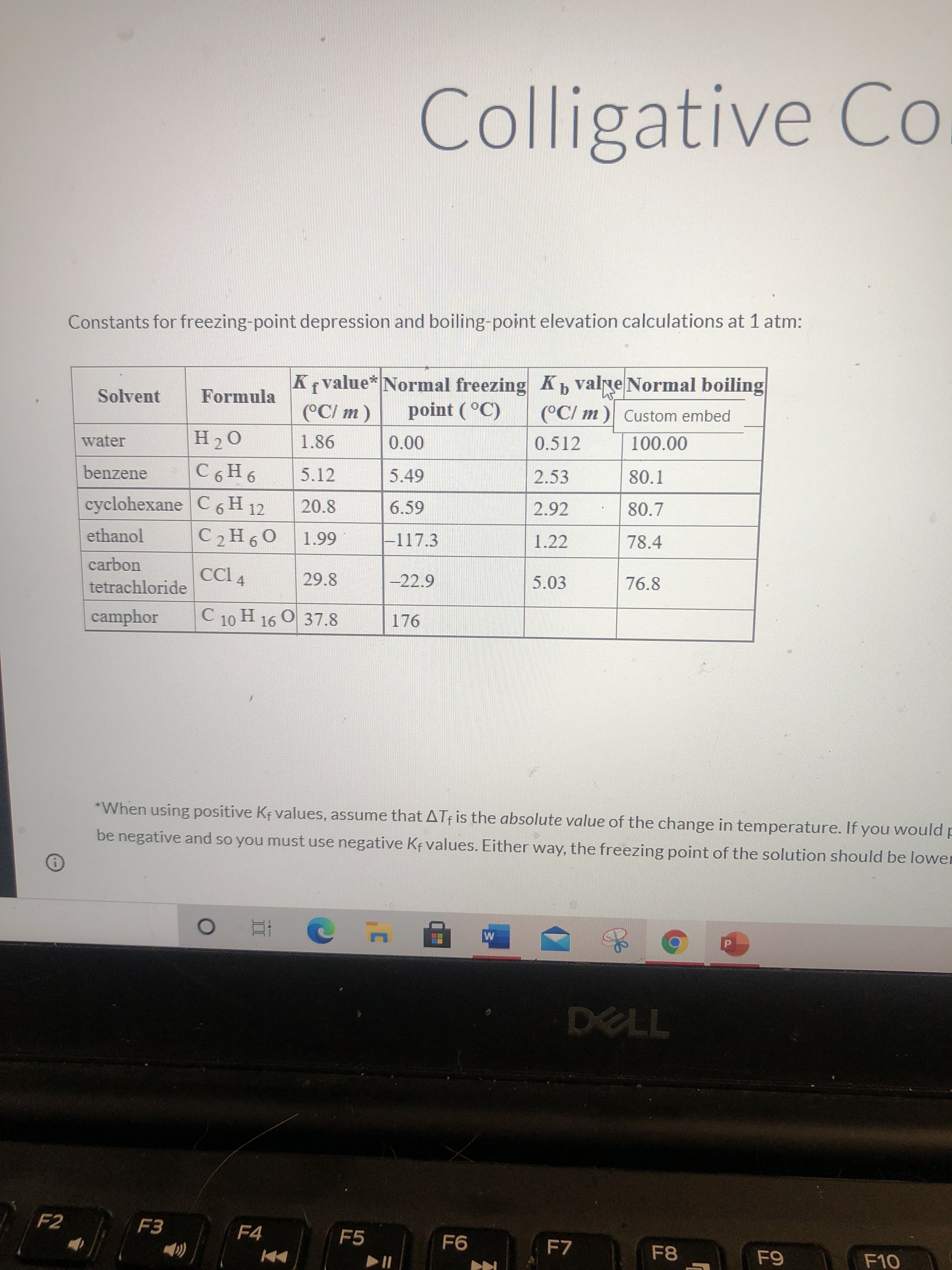
Calculate the boiling points of a 8.50 m aqueous solution of fructose. Boiling point constants can be found in the list of constant.
Tb= In Celsius
Transcribed Image Text:LL
LL
Colligative Co
Constants for freezing-point depression and boiling-point elevation calculations at 1 atm:
K Kb valne Normal boiling
value* Normal freezing
Solvent
Formula
(°C/ m)
point (°C)
(°C/ m ) Custom embed
0.512
00 0
5.49
water
00'001
benzene
5.12
2.53
80.1
cyclohexane C6 H 12
20.8
6.59
2.92
80.7
ethanol
C2H60
66 T
-117.3
1.22
78.4
carbon
CCI 4
29.8
-22.9
5.03
76.8
tetrachloride
camphor
C 10 H 16 O 37.8
176
*When using positive Ke values, assume that ATf is the absolute value of the change in temperature. If you would p
be negative and so you must use negative Kf values. Either way, the freezing point of the solution should be lower
五。
F2
F4
F5
F7
F8
(
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you



