
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please explain
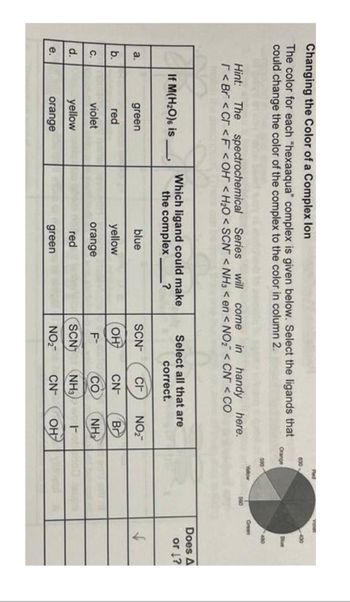
Transcribed Image Text:Changing the Color of a Complex lon
The color for each "hexaaqua" complex is given below. Select the ligands that
could change the color of the complex to the color in column 2.
a.
b.
C.
d.
e.
Hint: The spectrochemical Series will come in handy here.
r< Br< Cr <F <OH <H₂O <SCN <NH3 <en <NO₂ < CN <CO
If M(H₂O) is
green
red
violet
yellow
orange
Which ligand could make
the complex?
blue
yellow
orange
red
green
Select all that are
correct.
CH
NO₂™
CN
Br
CO
NH₂
SCN
NH3
F
NO₂™ CN он
SCN™
OH
F-
5:30
Orange
Fied
430
Green
Blun
Does A
or ?
↓
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- True or false if ring A was flipped it would result in ring B?arrow_forwardi) ОН MCPBA CH2Cl2 ОН H3PO4 heat D2O, D+arrow_forward(a) dispersion force Dispersion forces are (Very weak),(Very strong) electrostatic attractions that occur due to the random electronic motion within all substances,(excluding),(including) those that are nonpolar. When the electrons within a molecule or atom are distributed asymmetrically about the nucleus, that molecule or atom will adopt a (temporary),(permanent), (induced),(instantaneous) dipole. The presence of this dipole can then distort the electrons of a neighboring atom or molecule, producing an (induced),(instantaneous) dipole. These two rapidly fluctuating dipoles thus result in a brief electrostatic (attraction),(Repulsion) between the two species. These forces are stronger in (Larger and heavier),(Smaller and lighter) atoms and molecules. For example, dispersion forces between (Fluorine),(iodine) molecules will be stronger than dispersion forces between (Fluorine),(iodine) molecules. (b) dipole-dipole attraction A dipole-dipole force is the electrostatic attraction…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY