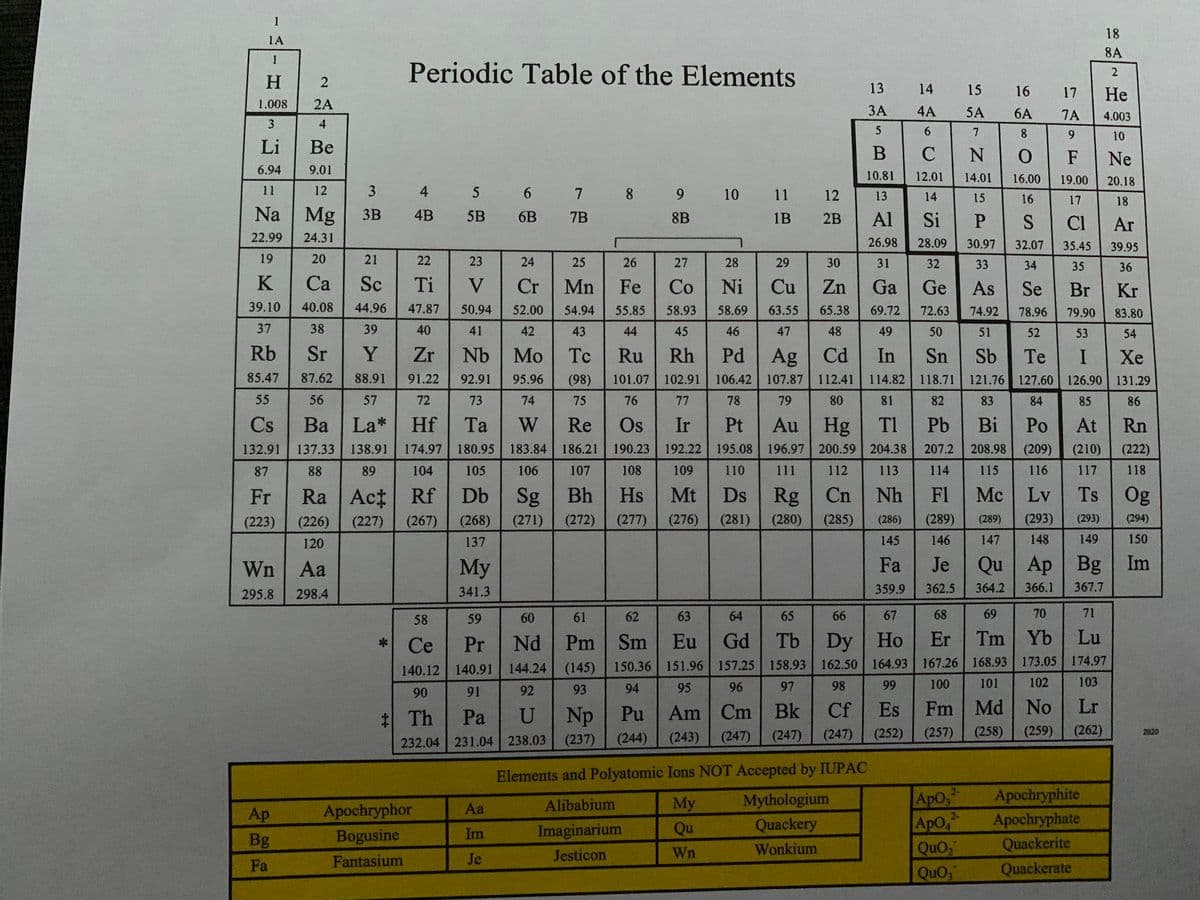
You may need to use a special periodic table to answer the question, it is attached in the images below. You will also need to look at the images to see the figure for the dot sturcture. a) Draw the electron dot structure for Fa. c) Would nitrogen have a larger or smaller ionization energy compared to Fa? (Please explain your answer)
You may need to use a special periodic table to answer the question, it is attached in the images below. You will also need to look at the images to see the figure for the dot sturcture. a) Draw the electron dot structure for Fa. c) Would nitrogen have a larger or smaller ionization energy compared to Fa? (Please explain your answer)
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter31: Immunochemistry
Section: Chapter Questions
Problem 31.75P
Related questions
Question
Quesion 10- (Please showall of your work so I can understand going forward)
You may need to use a special periodic table to answer the question, it is attached in the images below. You will also need to look at the images to see the figure for the dot sturcture.
a) Draw the electron dot structure for Fa.
c) Would nitrogen have a larger or smaller ionization energy compared to Fa? (Please explain your answer)
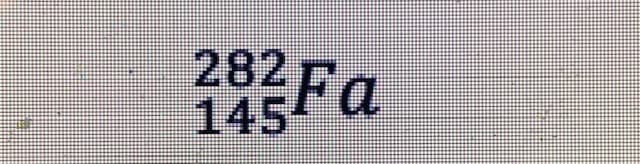
Transcribed Image Text:282 Fa
145
Transcribed Image Text:1
18
1A
8A
1
Periodic Table of the Elements
H.
13
14
15
16
17
Не
1.008
2A
ЗА
4A
5A
6A
7A
4.003
3
4
6.
7
8.
10
Li
Be
F
Ne
6.94
9.01
10.81
12.01
14.01
16.00
19.00
20.18
11
12
3
4
6.
7
9.
10
11
12
13
14
15
16
17
18
Na Mg
3B
4B
5B
6B
7B
8B
1B
2B
Al
Si
CI
Ar
22.99
24.31
26.98
28.09
30.97
32.07
35.45
39.95
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
K
Ca
Sc
Ti
V
Cr
Mn
Fe
Co
Ni
Cu
Zn
Ga
Ge
As
Se
Br
Kr
39.10
40.08
44.96
47.87
50.94
52.00
54.94
55.85
58.93
58.69
63.55
65.38
69.72
72.63
74.92
78.96
79.90
83.80
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
Rb
Sr
Y
Zr
Nb Mo
Tc
Ru
Rh
Pd
Ag Cd
In
Sn
Sb
Те
I.
Xe
85.47
87.62
88.91
91.22
92.91
95.96
(98)
101.07 102.91
106.42 107.87 112.41
114.82 118.71
121.76 127.60 126.90 131.29
55
56
57
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
Cs
Ba
Hf
Та
W
Re
Os
Ir
Pt
Au Hg
Tl
Pb
Bi
Ро
At
Rn
132.91
137.33
138.91
174.97
180.95 183.84 186.21
190.23 192.22 195.08 196.97 200.59 204.38
207.2
208.98 (209)
(210)
(222)
87
88
89
104
105
106
107
108
109
110
111
112
113
114
115
116
117
118
Fr
Ra Ac¢
Rf
Db
Sg
Bh
Hs
Mt
Ds
Rg
Cn
Nh
F1
Mc
Lv
Ts
Og
(223)
(226)
(227)
(267)
(268)
(271)
(272)
(277)
(276)
(281)
(280)
(285)
(286)
(289)
(289)
(293)
(293)
(294)
120
137
145
146
147
148
149
150
Wn Aa
My
Fa
Je
Qu Ap
Bg | Im
295.8
298.4
341.3
359.9
362.5
364.2
366.1
367.7
58
59
60
61
62
63
64
65
66
67
68
69
70
71
* Ce
Pr
Nd Pm Sm
Eu
Gd
Tb
Dy Ho
Er
Tm Yb
Lu
(145)
150.36 151.96 157.25 158.93
162.50 164.93 167.26 168.93 173.05 174.97
140.12 140.91
144.24
92
93
94
95
96
97
98
99
100
101
102
103
90
91
Pu
Am Cm Bk
Cf
Es
Fm Md No
Lr
Np
(244)
t Th
Pa
U
(252)
(257)
(258)
(259)
(262)
232.04 231.04 238.03
(237)
(243)
(247)
(247)
(247)
2020
Elements and Polyatomic Ions NOT Accepted by IUPAC
Apochryphite
Apochryphate
ApO,
ApO,
QuO
QuO
Mythologium
Quackery
Aa
Alibabium
My
Apochryphor
Bogusine
Ap
2-
Im
Imaginarium
Qu
Bg
Quackerite
Jesticon
Wn
Wonkium
Fa
Fantasium
Je
Quackerate
8.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning