II Rb Courses- Blackboard Learn > General Psychology - Fall 2021 A ALEKS - Griffin Barden - Learn s.com/alekscgi/x/Isl.exe/1o_u-IgNsikr7j8P3jH-IJczzdcvSCzsqTCIDqNGV3bKqhMfPmUcQ4ENkmiXn9QCwgeDPDkQ06ysZYWESPcekwL0-Qg619rekU7404HgFAGbEZaDrC O THERMOCHEMISTRY Understanding the definitions of heat and work A mixture of gaseous reactants is put into a cylinder, where a chemical reaction turns them into gaseous products. The cylinder has a piston that moves in or out, as necessary, to keep a constant pressure on the mixture of 1 atm. The cylinder is also submerged in a large insulated water bath. (See sketch at right.) From previous experiments, this chemical reaction is known to absorb 350. kJ of energy. The temperature of the water bath is monitored, and it is determined from this data that 295. kJ of heat flows into the system during the reaction. O exothermic Is the reaction exothermic or endothermic? O endothermic dn O Does the temperature of the water bath go up or down? имор о O' neither Does the piston move in or out? O neither O does work Does the gas mixture do work, or is work done on it? O work is done on it neither How much work is done on (or by) the gas mixture? Round your answer to 2 significant digits.
II Rb Courses- Blackboard Learn > General Psychology - Fall 2021 A ALEKS - Griffin Barden - Learn s.com/alekscgi/x/Isl.exe/1o_u-IgNsikr7j8P3jH-IJczzdcvSCzsqTCIDqNGV3bKqhMfPmUcQ4ENkmiXn9QCwgeDPDkQ06ysZYWESPcekwL0-Qg619rekU7404HgFAGbEZaDrC O THERMOCHEMISTRY Understanding the definitions of heat and work A mixture of gaseous reactants is put into a cylinder, where a chemical reaction turns them into gaseous products. The cylinder has a piston that moves in or out, as necessary, to keep a constant pressure on the mixture of 1 atm. The cylinder is also submerged in a large insulated water bath. (See sketch at right.) From previous experiments, this chemical reaction is known to absorb 350. kJ of energy. The temperature of the water bath is monitored, and it is determined from this data that 295. kJ of heat flows into the system during the reaction. O exothermic Is the reaction exothermic or endothermic? O endothermic dn O Does the temperature of the water bath go up or down? имор о O' neither Does the piston move in or out? O neither O does work Does the gas mixture do work, or is work done on it? O work is done on it neither How much work is done on (or by) the gas mixture? Round your answer to 2 significant digits.
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.10QAP
Related questions
Question
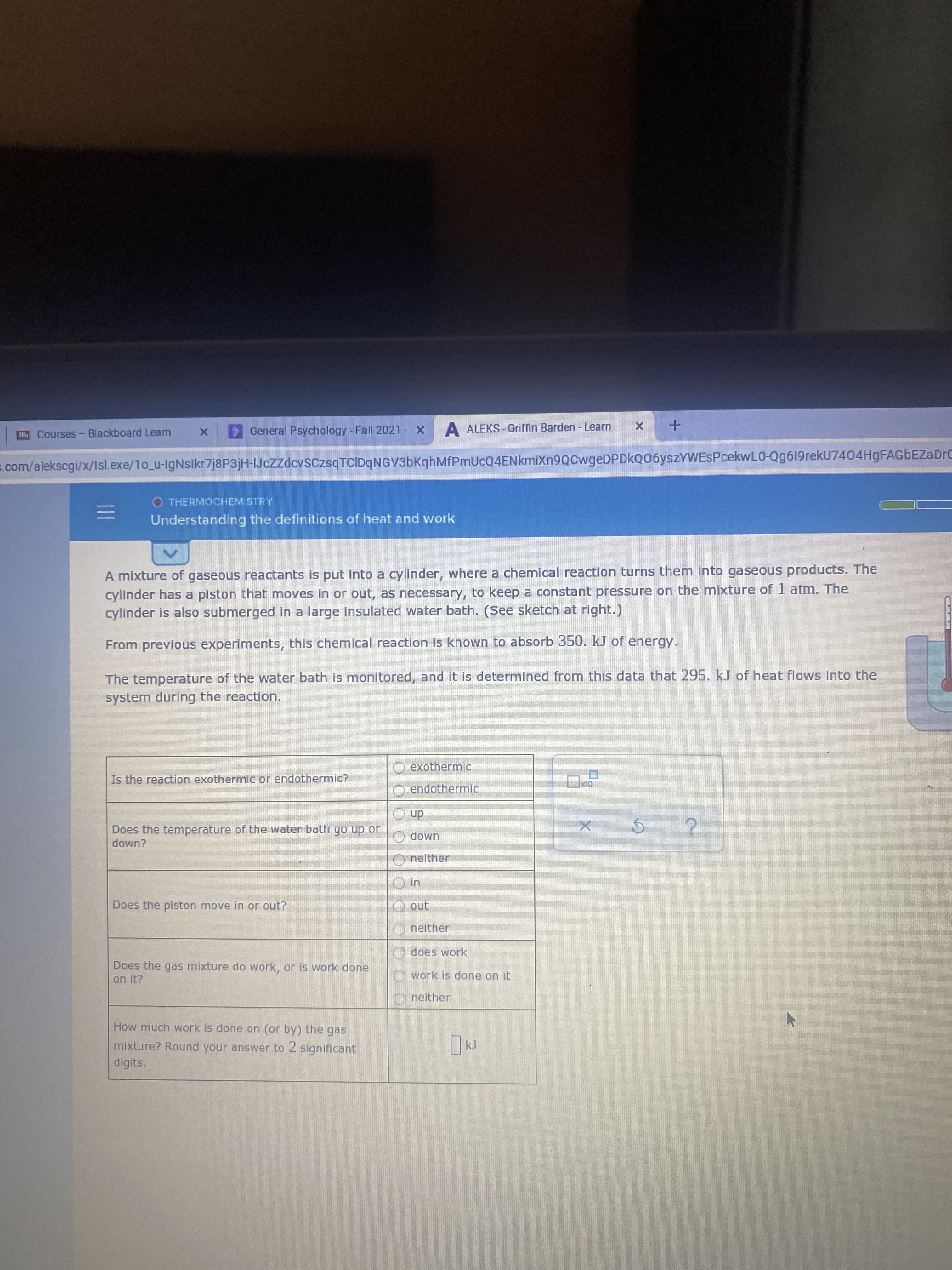
Transcribed Image Text:II
Rb Courses- Blackboard Learn
> General Psychology - Fall 2021
A ALEKS - Griffin Barden - Learn
s.com/alekscgi/x/Isl.exe/1o_u-IgNsikr7j8P3jH-IJczzdcvSCzsqTCIDqNGV3bKqhMfPmUcQ4ENkmiXn9QCwgeDPDkQ06ysZYWESPcekwL0-Qg619rekU7404HgFAGbEZaDrC
O THERMOCHEMISTRY
Understanding the definitions of heat and work
A mixture of gaseous reactants is put into a cylinder, where a chemical reaction turns them into gaseous products. The
cylinder has a piston that moves in or out, as necessary, to keep a constant pressure on the mixture of 1 atm. The
cylinder is also submerged in a large insulated water bath. (See sketch at right.)
From previous experiments, this chemical reaction is known to absorb 350. kJ of energy.
The temperature of the water bath is monitored, and it is determined from this data that 295. kJ of heat flows into the
system during the reaction.
O exothermic
Is the reaction exothermic or endothermic?
O endothermic
dn O
Does the temperature of the water bath go up or
down?
имор о
O' neither
Does the piston move in or out?
O neither
O does work
Does the gas mixture do work, or is work done
on it?
O work is done on it
neither
How much work is done on (or by) the gas
mixture? Round your answer to 2 significant
digits.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

