* CO • Part I Balance the reaction Cr2 O3 (s) + H2 (g) → Cr(s) + H2O(g). (8)0°H+(s)1Ɔ « Express your answer as a chemical equation. Identify all of the phases in your answer. A chemical reaction does not occur for this question. Submit Request Answer Part J Complete previous part(s) Part K tv ch or type URL 9. 8. * Co v Part K Balance the reaction Al(s) + Cl2 (g) AICI3 (s). Express your answer as a chemical equation. Identify all of the phases in your answer. DA chemical reaction does not occur for this question. Submit Request Answer Part L Complete previous part(s) Part M P Pearson Pearson Education Inc. All rights reserved. I Terms of Use l Privacy Policy Permissions Contact Us/ 100 Search or type URL 8. 6. 9.
* CO • Part I Balance the reaction Cr2 O3 (s) + H2 (g) → Cr(s) + H2O(g). (8)0°H+(s)1Ɔ « Express your answer as a chemical equation. Identify all of the phases in your answer. A chemical reaction does not occur for this question. Submit Request Answer Part J Complete previous part(s) Part K tv ch or type URL 9. 8. * Co v Part K Balance the reaction Al(s) + Cl2 (g) AICI3 (s). Express your answer as a chemical equation. Identify all of the phases in your answer. DA chemical reaction does not occur for this question. Submit Request Answer Part L Complete previous part(s) Part M P Pearson Pearson Education Inc. All rights reserved. I Terms of Use l Privacy Policy Permissions Contact Us/ 100 Search or type URL 8. 6. 9.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter16: Spontaneity Of Reaction
Section: Chapter Questions
Problem 85QAP: Which of the following quantities can be taken to be independent of temperature? independent of...
Related questions
Question
What would the balance reaction be for both of them?
Transcribed Image Text:* CO
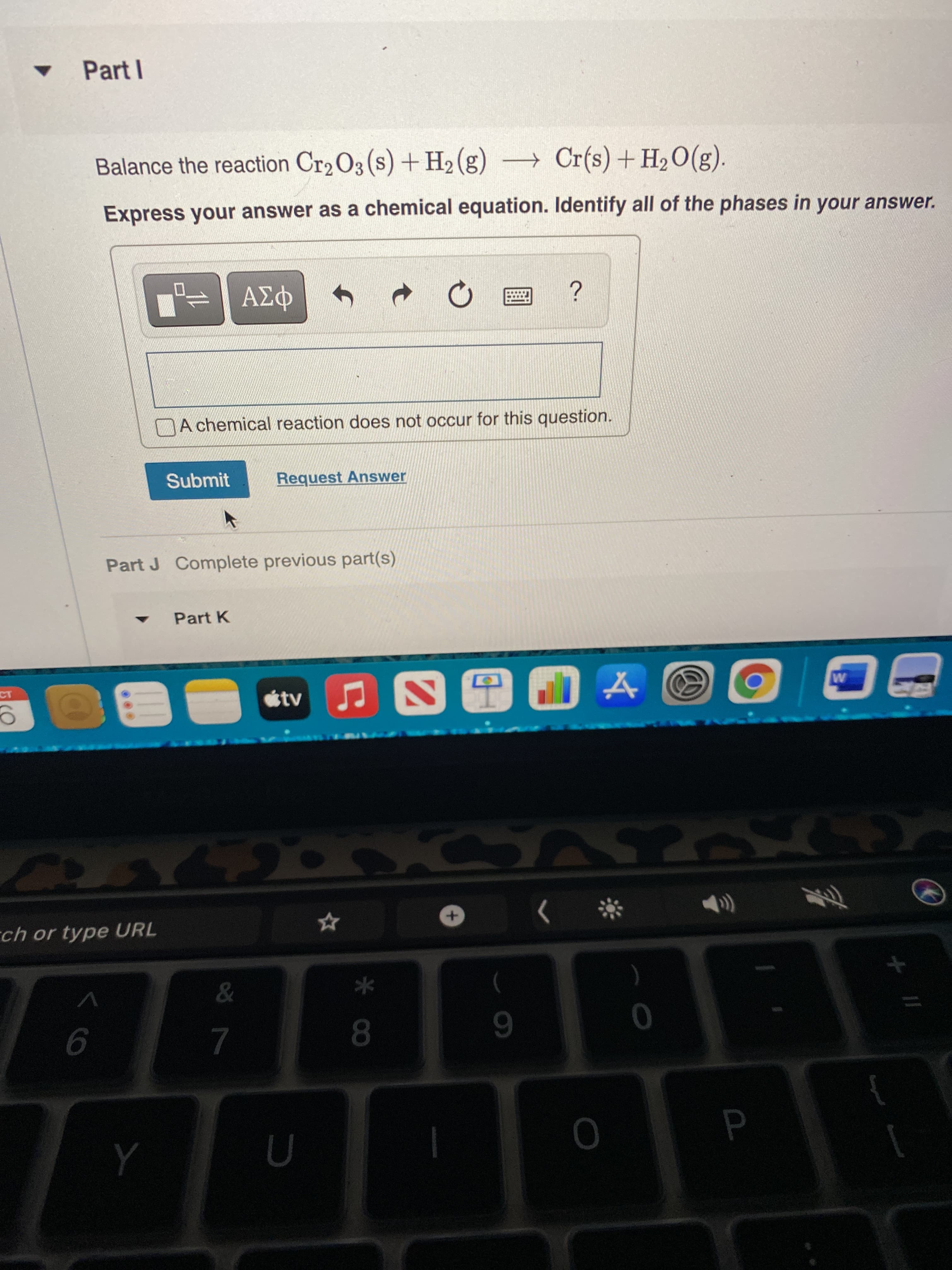
• Part I
Balance the reaction Cr2 O3 (s) + H2 (g) → Cr(s) + H2O(g).
(8)0°H+(s)1Ɔ «
Express your answer as a chemical equation. Identify all of the phases in your answer.
A chemical reaction does not occur for this question.
Submit
Request Answer
Part J Complete previous part(s)
Part K
tv
ch or type URL
9.
8.
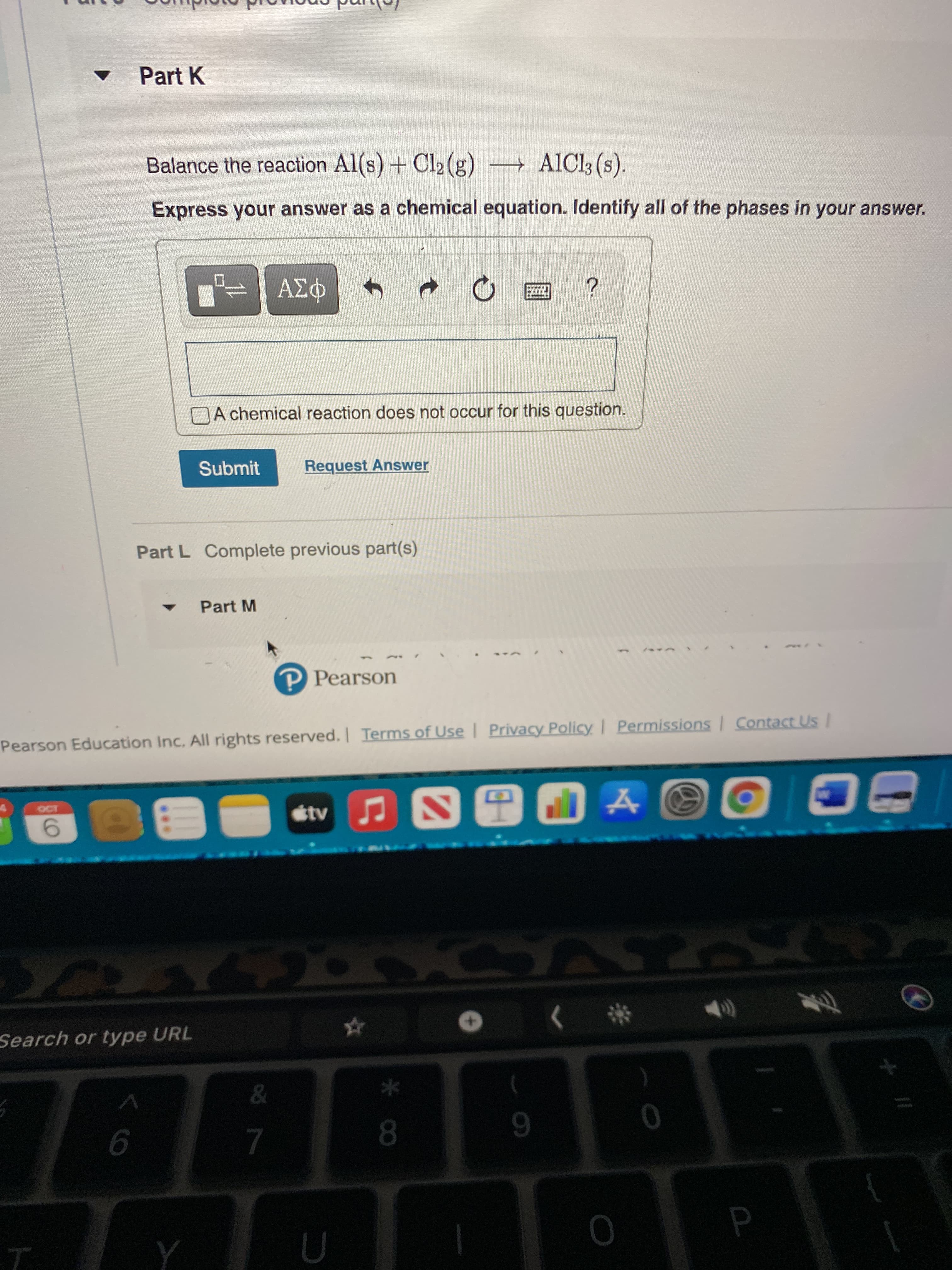
Transcribed Image Text:* Co
v Part K
Balance the reaction Al(s) + Cl2 (g) AICI3 (s).
Express your answer as a chemical equation. Identify all of the phases in your answer.
DA chemical reaction does not occur for this question.
Submit
Request Answer
Part L Complete previous part(s)
Part M
P Pearson
Pearson Education Inc. All rights reserved. I Terms of Use
l
Privacy Policy Permissions Contact Us/
100
Search or type URL
8.
6.
9.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning