4. 5. 3. 5.How would the experimental (actual)molar concentration for C12H22011 differ from the calculated concentration (higher, lower, or the same) given the following scenarios? Explain. (Hint: Compare the calculated concentrations to experimental concentrations). a. A student spilled some of the solid C12H22011 before adding it to the 50-mL volumetric flask. B.A student added water so that is was about 5 mm higher than the indicated line on the volumetric flask. SalWhen preparing the dilutedsolution, a student used the pipet bulb to blow out the last few drops in the pipet into the solution. Display Settir F3 F4 F5 F7 F11 F12
4. 5. 3. 5.How would the experimental (actual)molar concentration for C12H22011 differ from the calculated concentration (higher, lower, or the same) given the following scenarios? Explain. (Hint: Compare the calculated concentrations to experimental concentrations). a. A student spilled some of the solid C12H22011 before adding it to the 50-mL volumetric flask. B.A student added water so that is was about 5 mm higher than the indicated line on the volumetric flask. SalWhen preparing the dilutedsolution, a student used the pipet bulb to blow out the last few drops in the pipet into the solution. Display Settir F3 F4 F5 F7 F11 F12
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.12QAP
Related questions
Question
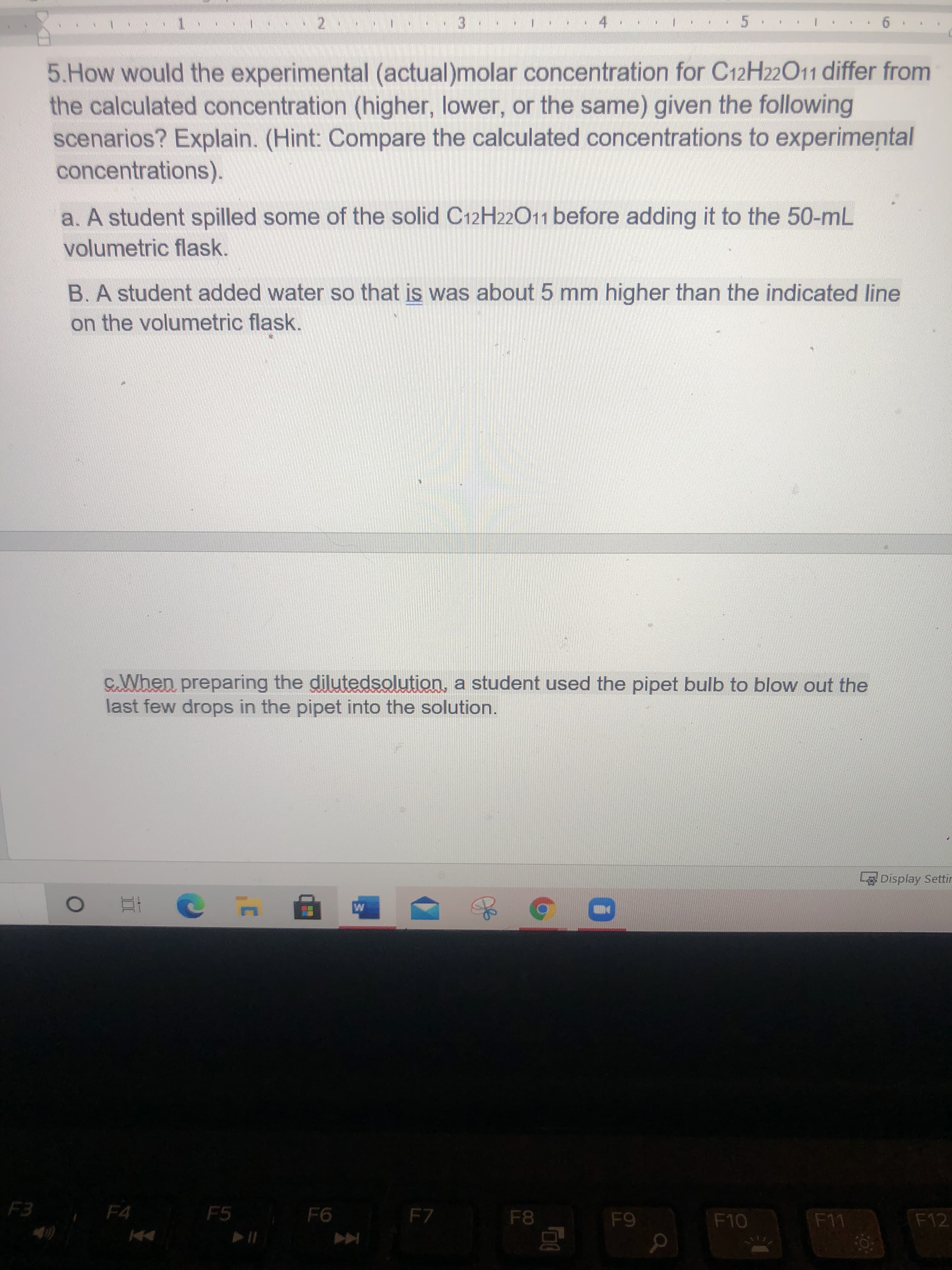
Transcribed Image Text:4.
5.
3.
5.How would the experimental (actual)molar concentration for C12H22011 differ from
the calculated concentration (higher, lower, or the same) given the following
scenarios? Explain. (Hint: Compare the calculated concentrations to experimental
concentrations).
a. A student spilled some of the solid C12H22011 before adding it to the 50-mL
volumetric flask.
B.A student added water so that is was about 5 mm higher than the indicated line
on the volumetric flask.
SalWhen preparing the dilutedsolution, a student used the pipet bulb to blow out the
last few drops in the pipet into the solution.
Display Settir
F3 F4
F5
F7
F11
F12
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you



