Rb Blackboard Collaborate Ultra -2 x General Psychology -Fall 20 How to Find a Career Path Using X V What Kind of Intelligence Do You X A ALEKS - Griffin Barden- Learn com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IJcZzdcvSCzsqTCIDqNGV3bKqhMfPmUcQ4ENkmiXn9QCwgeDPDkQ06yszYWESPcekwL0-Qg6l19rekU7404HgFAGBEZaDr080?1oBw7QYjlbavbSPXtx-YCjsh_7mMmrq O THERMOCHEMISTRY Griffin Calculating a molar heat of reaction from formation enthalpies Using the table of standard formation enthalpies that you'll find under the ALEKS Data tab, calculate the reaction enthalpy of this reaction under standard conditions: (6)°Hɔ-(5)
Rb Blackboard Collaborate Ultra -2 x General Psychology -Fall 20 How to Find a Career Path Using X V What Kind of Intelligence Do You X A ALEKS - Griffin Barden- Learn com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IJcZzdcvSCzsqTCIDqNGV3bKqhMfPmUcQ4ENkmiXn9QCwgeDPDkQ06yszYWESPcekwL0-Qg6l19rekU7404HgFAGBEZaDr080?1oBw7QYjlbavbSPXtx-YCjsh_7mMmrq O THERMOCHEMISTRY Griffin Calculating a molar heat of reaction from formation enthalpies Using the table of standard formation enthalpies that you'll find under the ALEKS Data tab, calculate the reaction enthalpy of this reaction under standard conditions: (6)°Hɔ-(5)
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.13QAP
Related questions
Question
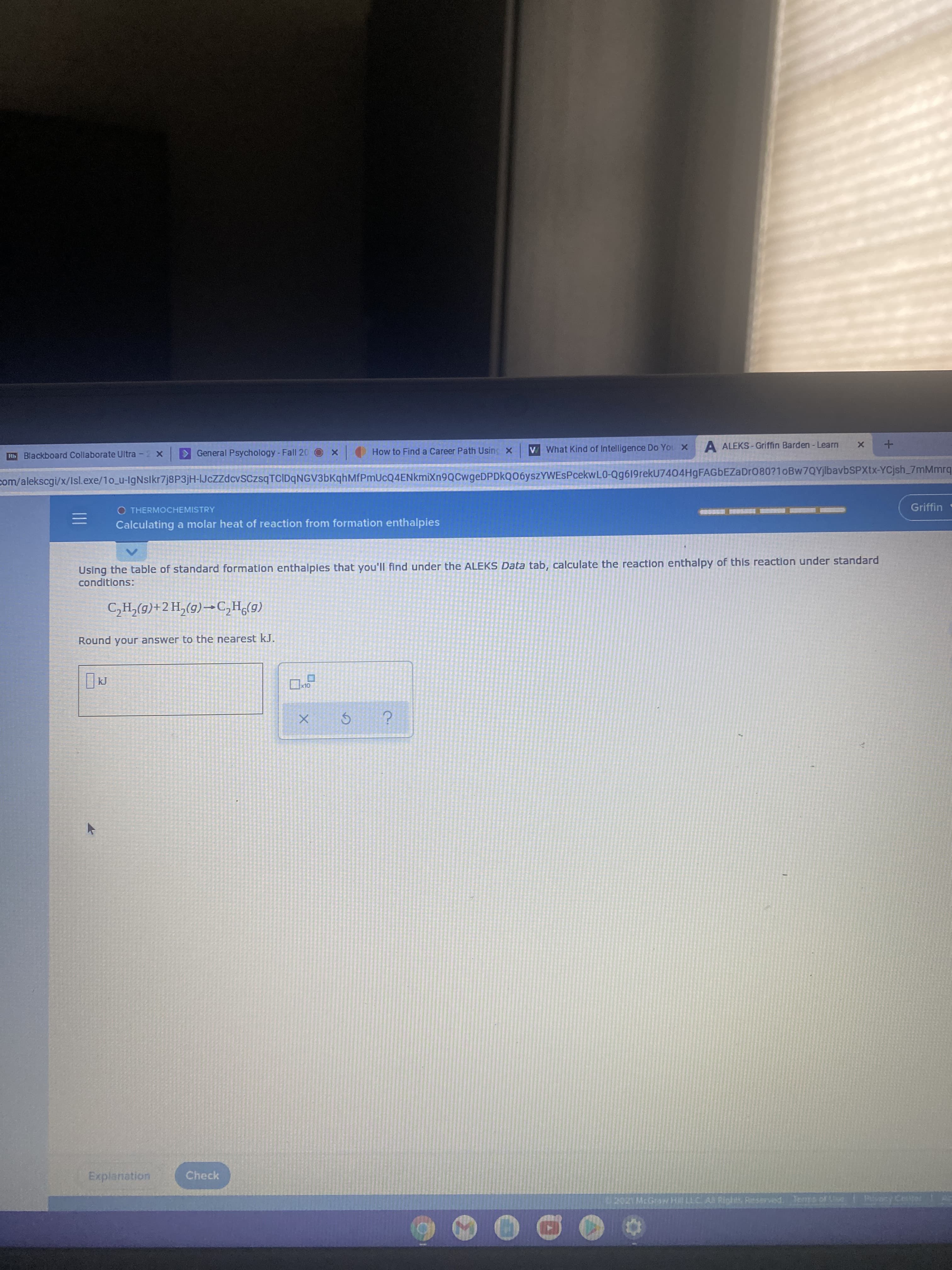
Transcribed Image Text:Rb Blackboard Collaborate Ultra -2 x
General Psychology -Fall 20
How to Find a Career Path Using X
V What Kind of Intelligence Do You X
A ALEKS - Griffin Barden- Learn
com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IJcZzdcvSCzsqTCIDqNGV3bKqhMfPmUcQ4ENkmiXn9QCwgeDPDkQ06yszYWESPcekwL0-Qg6l19rekU7404HgFAGBEZaDr080?1oBw7QYjlbavbSPXtx-YCjsh_7mMmrq
O THERMOCHEMISTRY
Griffin
Calculating a molar heat of reaction from formation enthalpies
Using the table of standard formation enthalpies that you'll find under the ALEKS Data tab, calculate the reaction enthalpy of this reaction under standard
conditions:
(6)°Hɔ-(5)<H7+(6)*H5
Round your answer to the nearest kJ.
OLX
Explanation
Check
U2021McGraw H LLC AN Riohts Reserved Terms of Use
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

