
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
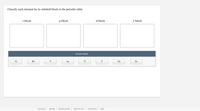
Transcribed Image Text:Classify each element by its subshell block in the periodic table.
s-block
p-block
d-block
f-block
Answer Bank
Fr
Be
P
Ag
Ti
F
Dy
Pu
about us
careers
privacy policy
terms of use
contact us
help
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which pair of elements do you expect to be most similar? Why?a) N and Nib) Mo and Snc) Na and Mgd) Cl and Fe) Si and Parrow_forwardWhich of the following statements are not true about the Rutherford Planetary Model? Loss of energy by an electron results in a change in position relative to the nucleus. Orbiting electrons are accelerating. Accelerating electrons give off electromagnetic radiation. Electrons increase distance from the nucleus after energy loss. Electrons follow an orbital path.arrow_forwardesc - a . 10 11 12 = 13 14 15 B B 16 17 18 19 20 21 22 23 24 26 27 28 29 30 31 32 33 or 25 :0 Y F1 1 F2 X N> A F5 8 T H|V R E 5 You will use the known periodic trends to compare properties for elements indicate with letters in this periodic table. Consider their relative positions for these questions. Your answers will be letters as indicated above, not the actual element symbol they would coordinate to. (Do not use a real periodic to answer this question.) • Which element would be expected to have the lowest first ionization energy? A • Which labeled element in the fourth period of the chart above has the highest quantity electron affinity energy? A MacBook Air DOO 80 DOO DII DD F3 F4 F6 F8 F9 F10 F7 A LIQ A 0 F11arrow_forward
- Choose the block of the periodic table where phosphorus is located. O s-block O f-block O outer block O d-block O p-blockarrow_forwardLook at the table you completed for Lab 3 and answer the questions below; Write down one pattern you’ve noticed occurring in your Table. Can you explain the reason for the pattern you observed in question 1 above? Look at the Periodic Table of elements on your Reference Table are there any other patterns you notice? Can you explain them?arrow_forwardPlease check and see if I got the information in the table below correct. If there are mistakes please correct them. Protons Neutrons Electrons Element Symbol Element Name Net Charge Mass Number Valence Electrons 1 0 1 H Hydrogen 0 1 1 6 6 6 C Carbon 0 12 4 8 8 8 O Oxygen 0 16 6 3 4 2 Li Lithium +1 7 0 5 6 4 B Boron +1 11 3 4 4 2 Be Beryllium +2 8 2 9 10 10 F Fluorine -1 19 8 2 2 2 He Helium 0 4 2 5 4 7 B Boron -2 9 5 6 6 6 C Carbon 0 12 4arrow_forward
- Select all of the elements that are members of the alkaline earth metals. calcium chlorine strontium bromine |iodine I sulfur | beryllium I rubidium I magnesium охyдen sodiumarrow_forwardEach element has a unique number of protons in its atoms * True Falsearrow_forwardWhat does the following element description actually mean? N+1 1. a sodium atom with 11 protons, 10 electrons, and 11 neutrons 2.a nitrogen atom with 6 protons, 14 electrons, and 7 neutrons 3.a sodium atom with 12 protons, 11 electrons, and 11 neutrons 4.a nitrogen atom with 7 protons, 6 electrons, and 7 neutrons 5.none of the abovearrow_forward
- Enter the number of electrons in each energy level (shell) for each of the elements. If the energy level does not contain any electrons, enter a 0. It may help to refer to the periodic table. H: n = 1 n = 2 n = 3 n = 4 O:arrow_forwardLook up the density of silicon and of lead in a table online. Then look up the atomic radius of silicon and lead . Then look up the atomic mass of each element (periodic table of elements). Use the atomic mass and atomic radius of each element to make an argument about density. Based on the atomic radii and atomic mass data, which element would be the most dense. Is that expectation consistent with actual density data? Use the definition of density to support your argument.arrow_forwardEnter the number of electrons in each energy level (shell) for each of the elements. If the energy level does not contain any electrons, enter a 0. It may help to refer to the periodic table. He n = 1 ______ n = 2 ______ n = 3______ n = 4______ F n = 1 _____ n = 2_____ n = 3 _____ n = 4______ P n = 1______ n = 2______ n = 3______ n = 4______ K n = 1_____ n = 2______ n = 3______ n = 4______ What is the neutral atom that has its first two energy levels filled, has 5 electrons in its third energy level, and has no other electrons? Enter the name of the element, not the abbreviation. Element name ________arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY