
Introductory Chemistry: A Foundation
9th Edition
ISBN: 9781337399425
Author: Steven S. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
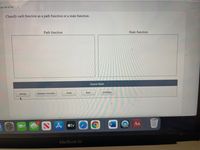
Transcribed Image Text:ion 34 of 41
<>
Classify each function as a path function or a state function.
Path function
State function
Answer Bank
work
heat
enthalpy
energy
distance traveled
Aa
A étv
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The heat of neutralization, Hneut, can be defined as the amount of heat released (or absorbed), q, per mole of acid (or base) neutralized. Hneut for nitric acid is -52 kJ/mol HNO3. At 27.3C, 50.00 mL of 0.743M HNO3 is neutralized by 1.00 M Sr(OH)2 in a coffee-cup calorimeter. (a) How many mL of Sr(OH)2 were used in the neutralization? (b) What is the final temperature of the resulting solution? (Use the assumptions in Question 11.)arrow_forward9.95 How much heat is required to convert 250 g of water from liquid at 23°C: into steam at 107°C? (The necessary heat capacities and enthalpy changes can be found in the chapter or online.)arrow_forwardDefine the following terms: potential energy, kinetic energy, path-dependent function, state function, system, surroundings.arrow_forward
- Starting with equation 2.27 andthe original definitionof enthalpy, derive the fact that Cp-=Cv-+Rarrow_forwardWhen ethanol (grain alcohol, is burned in oxygen, approximately 1360 kJ of heat energy is released per mole of ethanol. mg src=Images/HTML_99425-10-44QAP_image002.jpg alt="" align="top"/> What quantity of heat is released for each gram of ethanol burned? i>What is for the reaction as written? How much heat is released when sufficient ethanol is burned so as to produce 1 mole of water vapor?arrow_forward9.102 A runner generates 418 kJ of energy per kilometer from the cellular oxidation of food. The runner's body must dissipate this heat or the body will overheat. Suppose that sweat evaporation is the only important cooling mechanism. If you estimate the enthalpy of evaporation of water as 44 kJ/mol and assume that sweat can he treated as water, describe how you would estimate the volume of sweat that would have to be evaporated if the runner runs a 10-km race.arrow_forward
- Equal masses of liquid A, initially at 100C, and liquid B, initially at 50C, are combined in an insulated container. The final temperature of the mixture is 80C. All the heat flow occurs between the two liquids. The two liquids do not react with each other. Is the specific heat of liquid A larger than, equal to, or smaller than the specific heat of liquid B?arrow_forwardHow many mL of water at 10C (2 significant figures) must be added to 75 mL of water at 35C to obtain a final temperature of 19C? (Make the same assumptions as in Question 9.)arrow_forward9.87 What will be the final temperature of a mixture made from equal masses of the following: water at 25.0 C, ethanol at 35.5 C, and iron at 95.0 C?arrow_forward
- Difference between the system and the surroundings. Give examples of both.arrow_forwardOn complete combustion at constant pressure, a 1.00-L sample of a gaseous mixture at 0C and 1.00 atm (STP) evolves 75.65 kJ of heat. If the gas is a mixture of ethane (C2H6) and propane (C3H8), what is the mole fraction of ethane in the mixture?arrow_forwardA piston performs work of 210. L atm on the surroundings, while the cylinder in which it is placed expands from 10. L to 25 L. At the same time, 45 J of heat is transferred from the surroundings to the system. Against what pressure was the piston working?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning