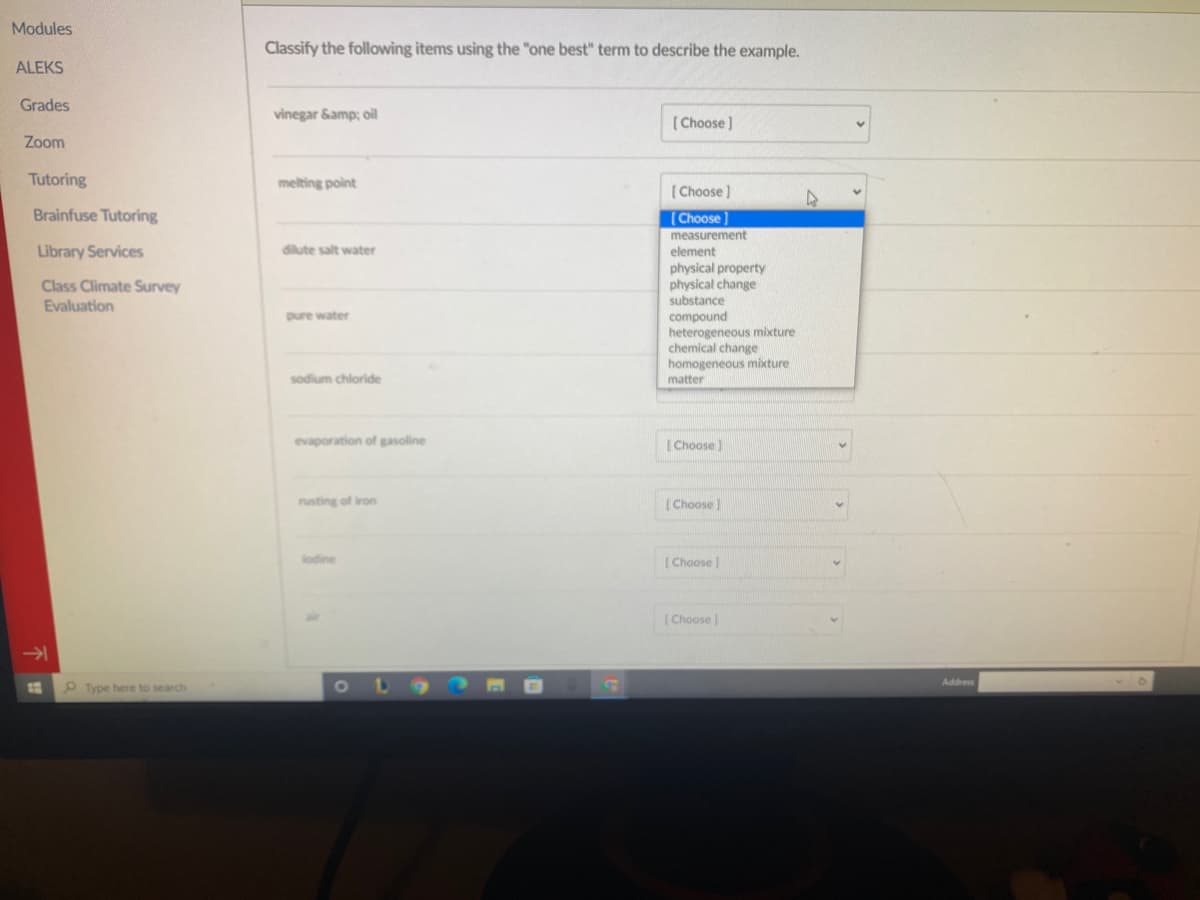
Classify the following items using the "one best" term to describe the example. vinegar Samp; oil ( Choose ) melting point ( Choose) (Choose measurement element dilute salt water physical property physical change substance pure water compound heterogeneous mixture chemical change homogeneous mixture sodium chloride matter evaporation of gasoline | Choose ) rusting of iron [Choose [ Choose (Choose
Classify the following items using the "one best" term to describe the example. vinegar Samp; oil ( Choose ) melting point ( Choose) (Choose measurement element dilute salt water physical property physical change substance pure water compound heterogeneous mixture chemical change homogeneous mixture sodium chloride matter evaporation of gasoline | Choose ) rusting of iron [Choose [ Choose (Choose
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 29P
Related questions
Question
Transcribed Image Text:physicar
Modules
Classify the following items using the "one best" term to describe the example.
ALEKS
Grades
vinegar Samp; oil
( Choose )
Zoom
Tutoring
melting point
( Choose )
Brainfuse Tutoring
[Choose
measurement
Library Services
diute salt water
element
physical property
I change
Class Climate Survey
Evaluation
substance
pure water
compound
heterogeneous mixture
chemical change
homogeneous mixture
sodium chloride
matter
evaporation of gasoline
( Choose)
rusting of iron
[Choose)
lodine
(Choose
(Choose
Address
P Type here to search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,