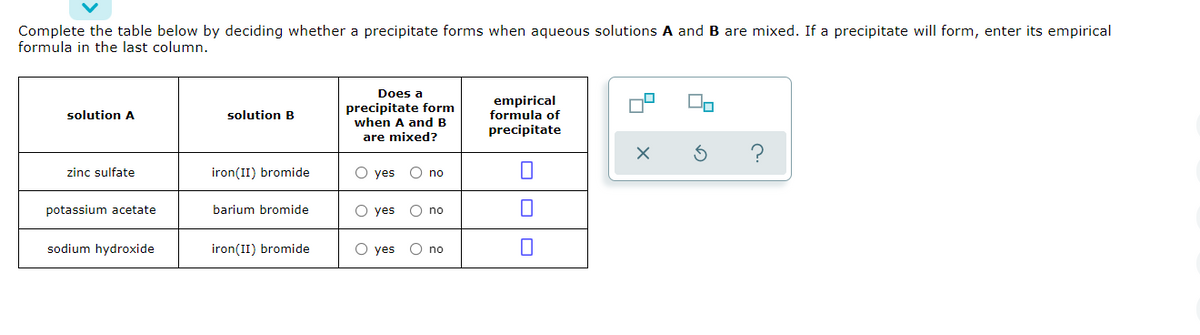
complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter it's empirical formula in the last column
Q: Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are…
A: For each solution A and B it is check whether precipitate forms or not and so if it forms emprical…
Q: precipitation reaction involves the formation of a precipitate when aqueous solutions are mixed. Not…
A: Balance Chemical equation means no of atoms or ions should be equaal in both side reactant and…
Q: Based on the solubility rules, which of these processes will occur if solutions of CuSO4 (aq) and…
A: Chemical precipitation is formation of separable solid substance from a solution, either by…
Q: Which of the following are examples of soluble compounds? Hydroxides Chromates Sulfides Nitrates
A: The compounds given are hydroxides, chromates, sulfides and nitrates.
Q: How many grams of nickel(II) chloride do you need to precipitate 503 mg of silver chloride in the…
A: given; In the reaction of nickel(II) chloride with silver nitrate: Note: (1 mg = 10-3 gram) Mass of…
Q: Write a balanced equation for the double-replacement precipitation reaction described, using the…
A: Here we are asked to write the balanced equation when aqueous solution of zinc iodide and potassium…
Q: Does a precipitate form when a solution of calcium chloride and a solution of mercury(I) nitrate are…
A: Here, we have to find whether a precipitate will form or not when a solution of calcium chloride and…
Q: write and balance: When aqueous iron (III) sulfate is mixed with aqueous magnesium hydroxide, iron…
A: Given: When aqueous iron (III) sulfate is mixed with aqueous magnesium hydroxide, iron (III)…
Q: . Solid sodium fluoride is slowly added to 125 mL of a 0.111 M calcium bromide solution until the…
A: The solubility product of salt is a constant at a given temperature irrespective of the source from…
Q: Based on the solubility rules, which of the following will occur if solutions of CuSO4(aq) and…
A:
Q: 471 mL of 2.013 M linthium baltimide (LiBr, 84.781 g/mol) is combined with 380 mL of 6.582 M shorium…
A: Calculation
Q: For the chemical equation 2HBr (aq) + Ba(OH)2 (aq) yields to 2H2O (L) + BaBr2 (aq) write the net…
A: Net ionic equation involves only the ions that actually take part in the reaction excluding the…
Q: Write a balanced equation for the double-replacement precipitation reaction described, using the…
A: Double displacement also known as metathesis reaction in which parts of two ionic compounds are…
Q: A reaction between a solution of sodium carbonate is mixed with a solution of calcium nitrate to…
A: Volume of Na2CO3 = 492.1 ml Molarity of Na2CO3= 0.674 mole/L Volume of Ca(NO3)2 = 551.4 ml Molarity…
Q: A sample of an unknown sulfate compound has a mass of 0.100 g. Addition of excess barium chloride…
A: A question based on percentage mass, which is to be accomplished.
Q: Based on the solubility rules, which of the following will occur when solutions of ZnSO4(aq) and…
A: From solubility rules no precipitate will form after the reaction of ZnSO4 and MgCl2.
Q: A student determines the chromium(III) content of a solution by first precipitating it as…
A:
Q: Calcium in river water samples can be precipitated out as Ca10(PO4)6(OH)2 (1,004.6 g/mol). The…
A: IMPORTANT POINTS Approximate value of Calcium present in nearby laboratory water is given in the…
Q: Distinguish among the terms solution,solute, and solvent.
A: A solution is a homogeneous mixture of two or more substances and the particle size is usually…
Q: The solubility differs from concentration because concentration is amount of solute per volume of…
A: Solution is the homogeneous mixture of two or more substances which consists of two phases solute…
Q: Write a balanced equation for the double-replacement precipitation reaction described, using the…
A: Double-displacement reaction defined as a type of chemical reaction in which the two reactants…
Q: Write a balanced equation for the double-replacement precipitation reaction described, using the…
A: Since, We know that, Balanced equation means number of atom in reactant side is equal to number of…
Q: Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are…
A: The solutions given are,
Q: Write a balanced equation for the double-replacement precipitation reaction described, using the…
A: A precipitate calcium sulphate forms when aqueous solutions of calcium bromide and potassium…
Q: Write a balanced equation for the double-replacement precipitation reaction described, using the…
A: In a double replacement precipitation reaction the cation and anion of both the reactants are…
Q: Complete and balance the precipitation reactions. Include physical states. Refer to the solubility…
A:
Q: Given the following list, how many of the compounds are soluble in water? sodium nitrate ammonium…
A: (i) Nitrates are usually soluble(ii) salts that contain ammonium ions are soluble(iii) Carbonates…
Q: Use the solubility generalizations on the information page to predict if one or more precipitates…
A: Precipitate is an undissolved solid which is formed during some chemical double displacement…
Q: Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are…
A: We have given that Complete the table below by deciding whether a precipitate forms when aqueous…
Q: Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are…
A: Given table is : Complete the table and deciding whether precipitates forms when aqueous solutions…
Q: Write a balanced chemical equation based on the following description: solid barium carbonate…
A: A chemical reaction is symbolic representation of the conversion of substances to new substances. In…
Q: Use the solubility generalizations on the information page to predict if one or more precipitates…
A: SOLUTION: Step 1: The metathesis reaction between Lead (II) nitrate and Iron (III) chloride is shown…
Q: A 25.0-g sample of a bright blue, crystalline solid is heated in the presence of air. The solid…
A: A decomposition reaction can be described as a reaction in which the compound breaks down into two…
Q: Precipitation is an example of what type of reaction? a. single replacement b. double replacement…
A: Precipitation reaction: precipitation reaction is the formation of an insoluble salt when two…
Q: What mass of aluminum hydroxide will precipitate
A:
Q: Differentiate between Electrolyte and Nonelectrolyte Solutions?
A: Electrolyte Non-electrolyte Electrolyte are…
Q: When you are presented with additional details, it can sometimes be a little more difficult to…
A: Bromine reacts to bromine to form aluminum bromide. The equation for the balanced chemical reaction…
Q: Complete the table below by deciding whether a precipitate form when aqueous solutions A and B are…
A: Reaction between iron (II) chloride and sodium sulfide will be as following :- FeCl2 + Na2S…
Q: How many grams of lead (IV) chloride would precipitate if a 240 g sample of nickel (II) chloride was…
A: In a balanced chemical reaction the reactants react and the products form in the molar ratio of…
Q: Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are…
A: Precipitation reaction can be defined as a reaction occur when cations and anions in an aqueous…
Q: A mixture of MgCl, and inert material is analyzed to determine the Mg content. First the mixture is…
A: The given reaction is a precipitation reaction in which the precipitate formed is AgCl. Steps…
Q: A student determines the calcium content of a solution by first precipitating it as calcium…
A: Precipitation of Calcium nitrate, Ca(NO3)2 + 2NaOH ---> Ca(OH)2 + 2NaNO3 Decomposition of…
Q: Determine the limiting reactant and theoretical yield of the solid when 10.00 mL of a 1.00 M sodium…
A: The reactants given are sodium sulfate i.e Na2SO4 and barium chloride i.e BaCl2. Hence the double…
Q: Write the balanced NET ionic equation for the reaction when aqueous calcium chloride and aqueous…
A: give reactionCaCl2(aq) + Pb(NO3)2(aq)→Ca(NO3)2(aq)+PbCl2(s)
Q: A (aq) + ....B(aq) Č(s)+ D (aq) Experimental Volume of B/ mL Mass of Run precipitate/g 1 1.2 5.40 2…
A:
Q: When the following aqueous solutions are mixed together, will a precipitate form? Sodium chloride…
A: 1) The reactants given are NaCl (aq) and KNO3 (aq) Hence the possible double displacement reaction…
Q: The compound sodium phosphate is a strong electrolyte. Write the reaction when solid sodium…
A: A strong electrolyte can 100% or completely dissociate in water into its respective poly-ions. While…
Q: Write a balanced equation for the double-replacement precipitation reaction described, using the…
A: Welcome to bartleby Answer to the question is given below
Q: Write a balanced equation for the double-replacement precipitation reaction described, using the…
A: The equation of chemical reaction, which have a same number of atoms for each elements and total…
Q: Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are…
A: Whether the mixture of solution A and B forms precipitate or not it is to be determined and if the…
complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter it's empirical formula in the last column.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

- The ideal product of gravimetric analysis called precipitate should portray some specific properties. Which among the following is a property of a good precipitate and why?While working in a metal processing facility, Letlen had accidentally mixed two metal vatstogether creating an alloy. One vat was labeled for cadmium, while the other was not. It canbe assumed that these are of pure metal composition. To identify this metal, Letlen took 1.000 g of the homogenous alloy sample composed ofcadmium and the unknown metal, dissolved, and diluted it to exactly 100.0 mL in avolumetric flask. A 20.00-mL aliquot was taken and titrated this using 22.82 mL of 0.05000M EDTA. In a second 20.00-mL aliquot, the Cd was masked through the addition of HCN/NaCN buffer.The titration of the unknown metal in the aliquot required 15.13 mL of EDTA.MW: Cd (112.411 g/mol) a. Calculate the moles of Cd and the moles of unknown metal in the 20.00-mL aliquot.b. Calculate the moles of Cd and the moles of unknown metal in the sample.The arsenic in a 1.22-g sample of a pesticide was converted toAsO43- by suitable chemical treatment. It was then titratedusing Ag+ to form Ag3AsO4 as a precipitate. (a) What is theoxidation state of As in AsO43-? (b) Name Ag3AsO4 by analogyto the corresponding compound containing phosphorusin place of arsenic. (c) If it took 25.0 mL of 0.102 M Ag+to reach the equivalence point in this titration, what is themass percentage of arsenic in the pesticide?
- 25 mL of a bleach (NaOCl) sample is diluted to 500 mL. An excessive amount of KI is added to the 20 mL solution taken from here, and the released I2 is titrated with 35.5 mL of 0.0409 M NaS2O3. Accordingly, calculate the weight percent by volume of NaOCl in the sample.(MaNaOC:74,4 g/mol)It is required to know the concentration of an aqueous solution of H2SO4 that appeared in the laboratoryChemistry III and it's unlabeled. To this end, a student of analytical chemistry carried out the followingProcedure: He took 5.00 mL of a fresh and standardized solution of 0.525M NaOH and brought them to a250.0 mL balloon to be completed with distilled water. Subsequently, he poured 15.00 mL of the solutionH2SO4 of unknown concentration in an Erlenmeyer flask and added 2 drops of phenolphthalein.Using a burette filled with the last NaOH solution, he noticed that when adding 39.40 mL of the hydroxidethe Erlenmeyer solution reached a faint but permanent pink. With the above dataDetermine the concentration and pH of the H2SO4 solution.As part of a soil analysis on a plot of land, a scientist wants to determine the ammonium content using gravimetric analysis with sodium tetraphenylborate, Na+B(C6H5)4−. Unfortunately, the amount of potassium, which also precipitates with sodium tetraphenylborate, is non‑negligible and must be accounted for in the analysis. Assume that all potassium in the soil is present as K2CO3 and all ammonium is present as NH4Cl. A 5.095 g soil sample was dissolved to give 0.500 L of solution. A 150.0 mL aliquot was acidified and excess sodium tetraphenylborate was added to precipitate both K+ and NH4+ ions completely. B(C6H5)4-+K+⟶KB(C6H5)4(s) B(C6H5)4-+NH4+⟶NH4B(C6H5)4(s) The resulting precipitate amounted to 0.269 g. A new 300.0 mL aliquot of the original solution was made alkaline and heated to remove all of the NH4+ as NH3. The resulting solution was then acidified, and excess sodium tetraphenylborate was added to give 0.129 g of precipitate. Find the mass percentages of NH4Cl and…
- In order to prepare for a qualitative analysis experiment, Felix is predicting whether small samples of several pairs of 0.10 M control solutions will form a precipitate when mixed. He uses the table of solubility values provided as well as the general solubility guidelines in the chempendix. Solubilities of Alkaline Earth Salts (g/100 g H2O, at 20−25∘C) OH− CO2−3 SO2−4 CrO2−4 C2O2−4 Mg2+ 0.00069 0.18 35.7 54.8 0.038Ca2+ 0.16 0.00066 0.205 13.2 0.00061Sr2+ 2.25 0.00034 0.135 0.106 0.005Ba2+ 4.91 0.0014 0.00031 0.00026 0.0075For each pair of compounds, predict the formula for the precipitate that Felix will see when he mixes the solutions in lab. If no precipitate forms, enter NP for no precipitate.The mass of K3PO4 needed to prepare 250.0mL of an aqueous solution in which PO4-3 concentration is 0.0550M. The answer is ……………………… How many grams of silver sample is equal to 0.0417 mole of silver The answer is ……………………… When 38.0 mL of 0.1250 M H2SO4 is added to 100 mL of a solution of PbI2, a precipitate of PbSO4 forms. The PbSO4 is then filtered from the solution, dried, and weighed. If the recovered PbSO4 is found to have a mass of 0.0471g, what was the concentration of iodide ions in the original solution The answer is ……………………………… Express 96.342 m using 2 significant figures The answer is ………………………………. The oxidation number of sulfur in (Na2S2O5) is? The answer is ………………………………What wt of magnetite should be taken for analysis in order that after converting to a precipitate of Fe2O3.xH2O, the percentage of Fe3O4 in the sample can be found by multiplying the wt in grams of the ignited precipitate (Fe2O3) by 100.
- A sample of soluble salt weighs 1.2 g and contains chloride, bromide and iodide. With AgNO3, a precipitate is obtained which weighs 0.4500 g. On heating this precipitate with Cl2 gas, the AgBr and AgI are converted to AgCl, and the precipitate then weighs 0.3300 g. A similar sample, when treated with palladous chloride, precipitates only PdI2, and this precipitate weighs 0.0900 grams. (*)Find the approximate percentage of chlorine, bromine and iodine in the samplesuppose you have a solution that might contain any or all of the following cations: Cu2+, Ag+, Ba2+, and Mn2+. The addition of HBr cuases a precipitate to from. After the precipitate is separated by filtration, H2SO4 is added to the supernatant liquid, and another precipitate forms. Thsi precipitate is separated bu filtration and a solution of NaOH is added to the supernatant liquid until it si strongly alkaline. No precipitate is fromed.Which ions are present in each of the precipitates? Which cations are not present in the original solution?You choose to investigate some of the solubility guidelinesfor two ions , the chromate ion(CrO4)-2 and the oxalate ion (C2O4) - 2. You are given 0.01 Msolutions (A, B, C, D) of four water-soluble salts: When these solutions are mixed, the following observationsare made as given in table: (a) Write a net ionic equation for the reaction that occurs ineach of the experiments. (b) Identify the precipitate formed,if any, in each of the experiments.



