Complete the table below for the solutions of Cu2+ that you made and the given percent transmittance. Show the calculations below the table. Use A = 2-log (%T ) Concentration of Made Solutions Cu2+ (M) %T Absorbance 0.05 92.1 81.5 0.1 0.2 68.0 0.5 36.5
Complete the table below for the solutions of Cu2+ that you made and the given percent transmittance. Show the calculations below the table. Use A = 2-log (%T ) Concentration of Made Solutions Cu2+ (M) %T Absorbance 0.05 92.1 81.5 0.1 0.2 68.0 0.5 36.5
Chapter24: Introduction To Spectrochemical Methods
Section: Chapter Questions
Problem 24.16QAP
Related questions
Question
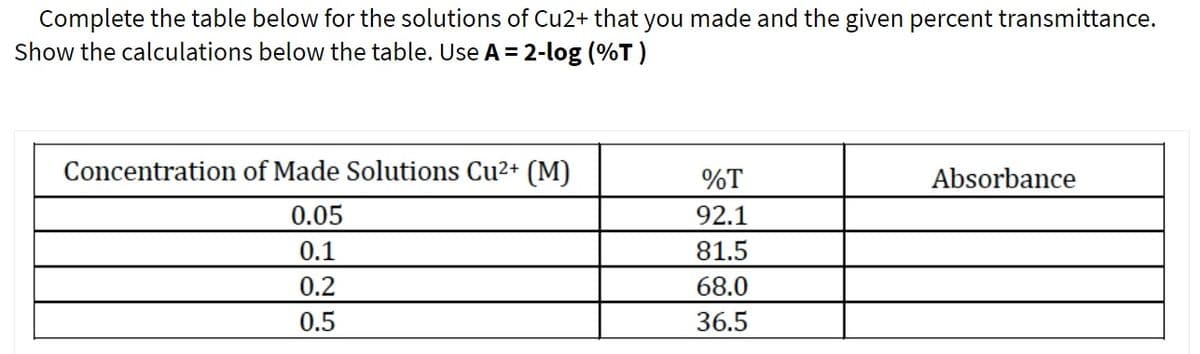
Transcribed Image Text:Complete the table below for the solutions of Cu2+ that you made and the given percent transmittance.
Show the calculations below the table. Use A = 2-log (%T )
Concentration of Made Solutions Cu2+ (M)
%T
Absorbance
0.05
92.1
81.5
0.1
0.2
68.0
0.5
36.5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning