Consider the molecule below, shown from two different perspectives. Which statement accurately describes the proton environments expected in 'H NMR? Br. CI Br CI H H H View 1 View 2 There are two distinct hydrogen environments. There is a plane of symmetry that can be drawn through the CH2 group. There are four distinct hydrogen environments. Two hydrogens are diastereotopic based on their positions relative to the halogen atoms; they cannot be interchanged by a symmetry reflection or rotation. O There are three distinct hydrogen environments. The CH2 hydrogens are equivalent because each one is adjacent to both a chlorine atom and a bromine atom.
Consider the molecule below, shown from two different perspectives. Which statement accurately describes the proton environments expected in 'H NMR? Br. CI Br CI H H H View 1 View 2 There are two distinct hydrogen environments. There is a plane of symmetry that can be drawn through the CH2 group. There are four distinct hydrogen environments. Two hydrogens are diastereotopic based on their positions relative to the halogen atoms; they cannot be interchanged by a symmetry reflection or rotation. O There are three distinct hydrogen environments. The CH2 hydrogens are equivalent because each one is adjacent to both a chlorine atom and a bromine atom.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter20: Dienes, Conjugated Systems, And Pericyclic Reactions
Section20.3: Uv-visible Spectroscopy
Problem 20.5P
Related questions
Question
100%
Answer this question and explain.
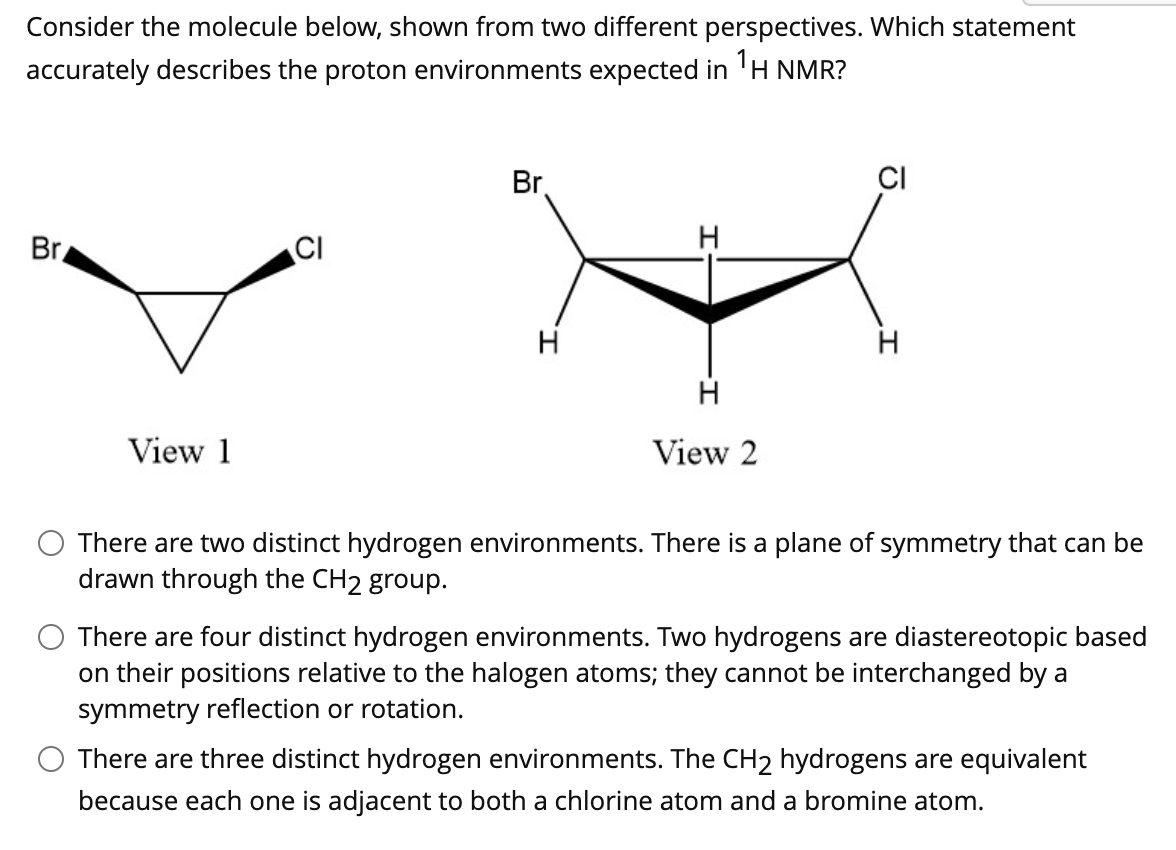
Transcribed Image Text:Consider the molecule below, shown from two different perspectives. Which statement
accurately describes the proton environments expected in 'H NMR?
Br
CI
Br
CI
H
H
H
View 1
View 2
There are two distinct hydrogen environments. There is a plane of symmetry that can be
drawn through the CH2 group.
There are four distinct hydrogen environments. Two hydrogens are diastereotopic based
on their positions relative to the halogen atoms; they cannot be interchanged by a
symmetry reflection or rotation.
There are three distinct hydrogen environments. The CH2 hydrogens are equivalent
because each one is adjacent to both a chlorine atom and a bromine atom.
Expert Solution
Step 1
NMR spectroscopy is a very important tool for the determination of the structure of the organic compound. The NMR spectrum provides the following details about the structure of the organic compound:
- The number of signals: The number of signals gives information about the different types of hydrogen atoms.
- Position of signal: The position of the signal tells us about the electronic environment of hydrogen.
- Splitting of signal: Splitting of the signal tells us about the number of neighboring hydrogen atoms present. If the number of the neighboring hydrogen atom is n then the signal splits in (n+1) peak.
- Area of signal: The area of the signal provides information about the number of hydrogen atoms responsible for the signal.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning