Constants |Fenodic Table You may want to reference (Pages 306 - 308) Section 9.5 while completing this problem. 18.0 mL of a 0.250 M KNO, solution from a 5.50 M KNO, solution Express your answer with the appropriate units. Determine the volume, in milliliters, required to prepare each of the following diluted solutions. ng HÀ Value Units > Submit Request Answer Part B 26.0 mL of a 2.50 M H2SO, solution from a(n) 11.8 MH2SO4 solution Express your answer with the appropriate units. HA Value Units Submit Request Answer P Pearson
Constants |Fenodic Table You may want to reference (Pages 306 - 308) Section 9.5 while completing this problem. 18.0 mL of a 0.250 M KNO, solution from a 5.50 M KNO, solution Express your answer with the appropriate units. Determine the volume, in milliliters, required to prepare each of the following diluted solutions. ng HÀ Value Units > Submit Request Answer Part B 26.0 mL of a 2.50 M H2SO, solution from a(n) 11.8 MH2SO4 solution Express your answer with the appropriate units. HA Value Units Submit Request Answer P Pearson
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 34E: Gold metal will not dissolve in either concentrated nitric acid or concentrated hydrochloric acid It...
Related questions
Question
100%
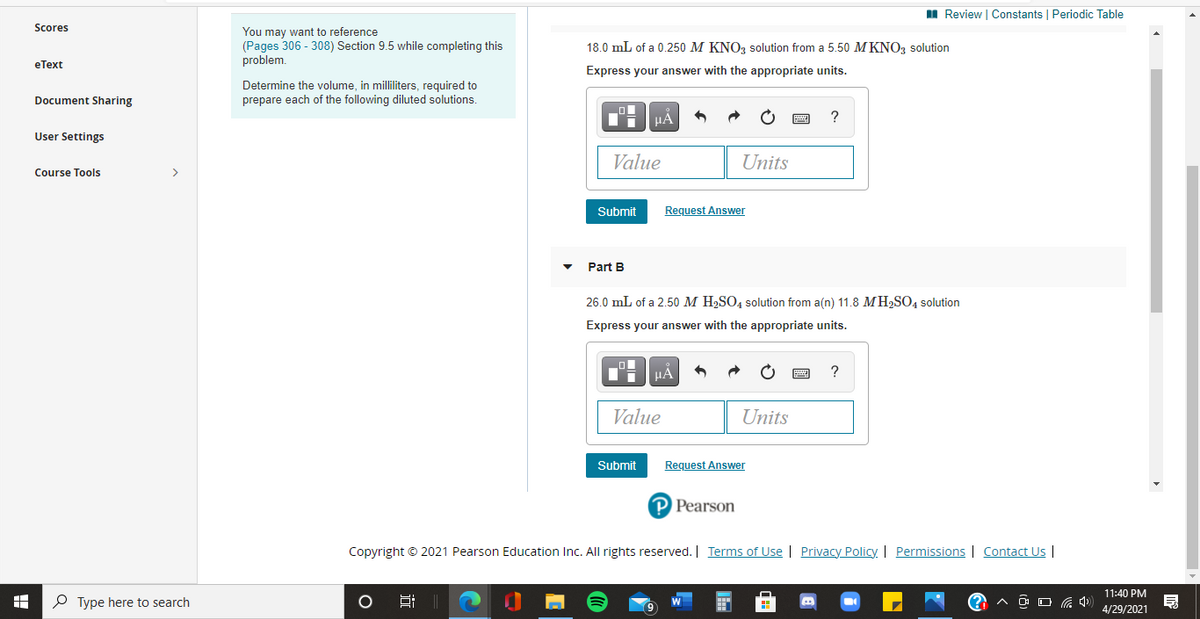
Transcribed Image Text:I Review | Constants | Periodic Table
Scores
You may want to reference
(Pages 306 - 308) Section 9.5 while completing this
problem.
18.0 mL of a 0.250 M KNO, solution from a 5.50 M KNO3 solution
eТext
Express your answer with the appropriate units.
Determine the volume, in milliliters, required to
prepare each of the following diluted solutions.
Document Sharing
TH HẢ
User Settings
Value
Units
Course Tools
>
Submit
Request Answer
Part B
26.0 mL of a 2.50 M H2SO4 solution from a(n) 11.8 MH2SO4 solution
Express your answer with the appropriate units.
?
Value
Units
Submit
Request Answer
P Pearson
Copyright © 2021 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy. | Permissions | Contact Us |
11:40 PM
P Type here to search
O O G 4)
4/29/2021
近
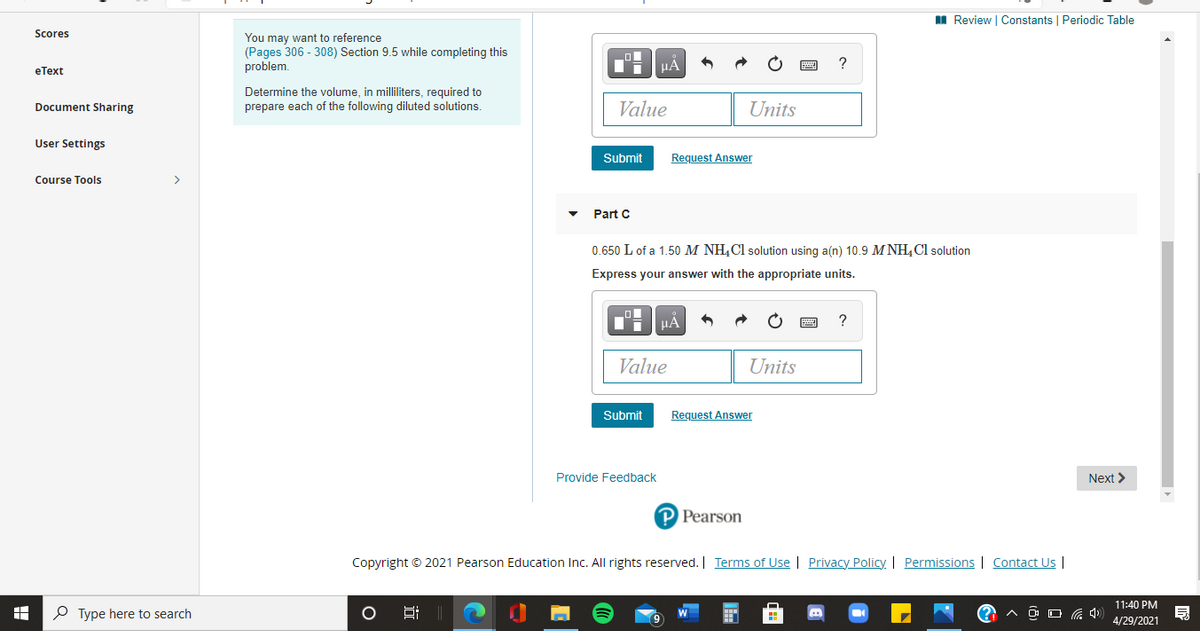
Transcribed Image Text:II Review | Constants | Periodic Table
Scores
You may want to reference
(Pages 306 - 308) Section 9.5 while completing this
problem.
HẢ
?
eТext
Determine the volume, in milliliters, required to
prepare each of the following diluted solutions.
Value
Units
Document Sharing
User Settings
Submit
Request Answer
Course Tools
>
Part C
0.650 L of a 1.50 M NH,Cl solution using a(n) 10.9 M NH,Cl solution
Express your answer with the appropriate units.
HÀ
Value
Units
Submit
Request Answer
Provide Feedback
Next >
P Pearson
Copyright © 2021 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy. | Permissions | Contact Us |
11:40 PM
P Type here to search
4/29/2021
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
