A student wanted to investigate the concentration of glucose in different energy drinks. The student first produced four glucose solutions of different, known concentrations by diluting a 4.0 mM glucose solution. The volumes of distilled water and glucose solution added to each test tube can be seen in Table 1. The total volume of each solution was 10 Table 1 Final concentration of glucose solution/ mM Volume of distilled water/ cm Volume of glucose soluti- 4.0 0.0 10.0 5.0 5.0 7.5 2.5 1.25 8.75 Complete Table 1 by filling in the three blank spaces with the correct final concentrations of glucose solution.
A student wanted to investigate the concentration of glucose in different energy drinks. The student first produced four glucose solutions of different, known concentrations by diluting a 4.0 mM glucose solution. The volumes of distilled water and glucose solution added to each test tube can be seen in Table 1. The total volume of each solution was 10 Table 1 Final concentration of glucose solution/ mM Volume of distilled water/ cm Volume of glucose soluti- 4.0 0.0 10.0 5.0 5.0 7.5 2.5 1.25 8.75 Complete Table 1 by filling in the three blank spaces with the correct final concentrations of glucose solution.
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter16: Solutions
Section: Chapter Questions
Problem 9E: What happens if you add a very small amount of solid salt (NaCl) to each beaker described below?...
Related questions
Question
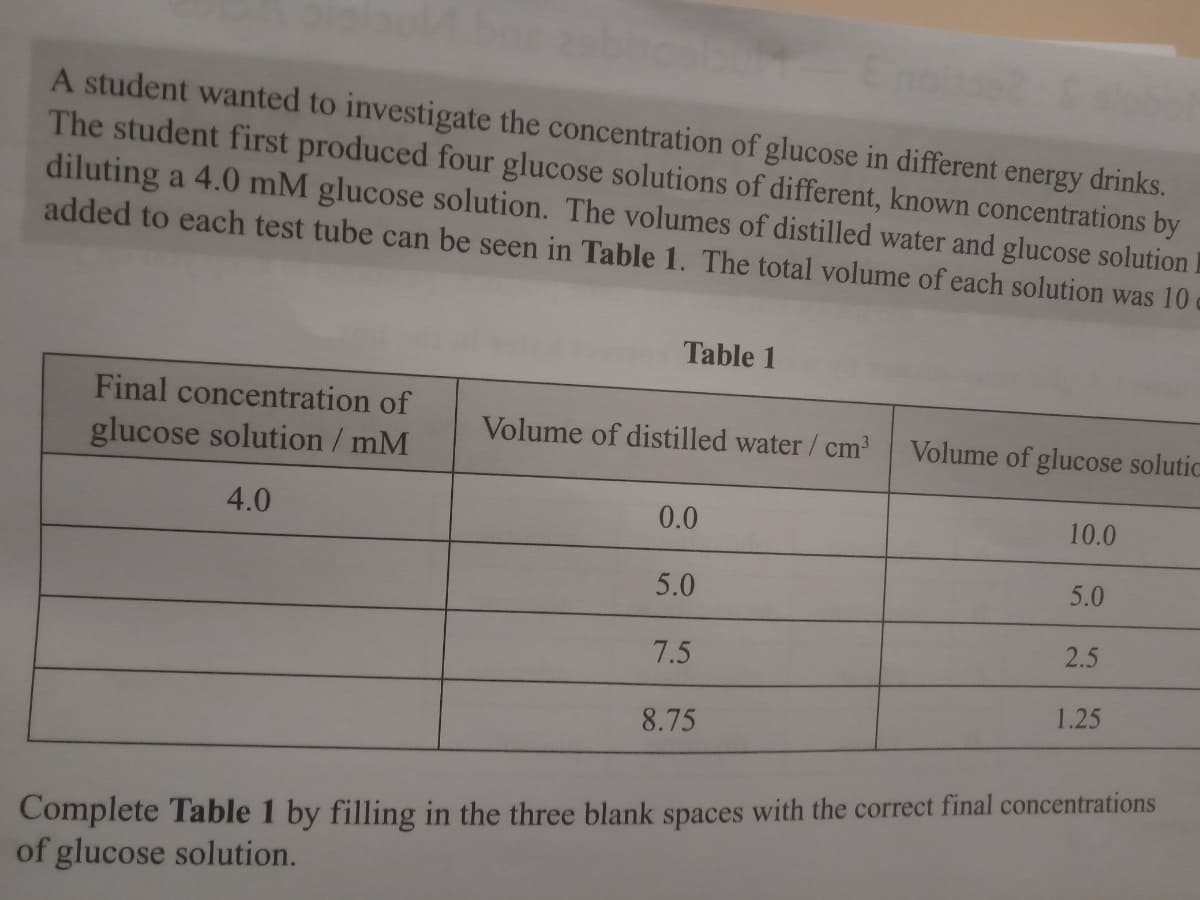
Transcribed Image Text:A student wanted to investigate the concentration of glucose in different energy drinks.
The student first produced four glucose solutions of different, known concentrations by
diluting a 4.0 mM glucose solution. The volumes of distilled water and glucose solution
added to each test tube can be seen in Table 1. The total volume of each solution was 10
Table 1
Final concentration of
glucose solution / mM
Volume of distilled water/ cm
Volume of glucose solutic
4.0
0.0
10.0
5.0
5.0
2.5
7.5
1.25
8.75
Complete Table 1 by filling in the three blank spaces with the correct final concentrations
of glucose solution.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning