Critical point 22,089 Water Ice (liquid) (solid) 101 Triple point 0.6 B. Water vapor (gas) A 0 0.01 100 374 Temperature (°C) Figure 10.31 The pressure and temperature axes on this phase diagram of water are not drawn to constant scale in order to illustrate several important properties. Pressure (kPa)
Critical point 22,089 Water Ice (liquid) (solid) 101 Triple point 0.6 B. Water vapor (gas) A 0 0.01 100 374 Temperature (°C) Figure 10.31 The pressure and temperature axes on this phase diagram of water are not drawn to constant scale in order to illustrate several important properties. Pressure (kPa)
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter6: Equilibria In Single-component Systems
Section: Chapter Questions
Problem 6.65E
Related questions
Question
Using the phase diagram for water given as shown, determine the state of water at the following temperatures and pressures:
(a) −10 °C and 50 kPa
(b) 25 °C and 90 kPa
(c) 50 °C and 40 kPa
(d) 80 °C and 5 kPa
(e) −10 °C and 0.3 kPa
(f) 50 °C and 0.3 kPa
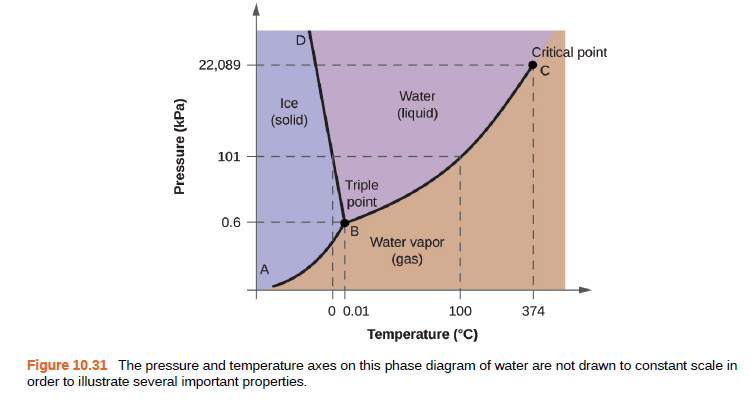
Transcribed Image Text:Critical point
22,089
Water
Ice
(liquid)
(solid)
101
Triple
point
0.6
B.
Water vapor
(gas)
A
0 0.01
100
374
Temperature (°C)
Figure 10.31 The pressure and temperature axes on this phase diagram of water are not drawn to constant scale in
order to illustrate several important properties.
Pressure (kPa)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 8 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning