Data Sheet II. Delivering Solution from Your Buret 17.28 (1) final buret reading, mL (2) initial buret reading, mL 6.52 (3) approved by 10.76 (4) volume of H;O delivered, mL (5) approved by VI. Preparing Your Bleaching Solution Sample Clorox (6) brand name of commercial bleaching solution 4.42 (7) cost of commercial bleaching solution per gallon (see laboratory instructor) 1.12 (8) density of commercial bleaching solution, g mL- (see instructor) (9) volume of commercial bleaching solution diluted to 100 mL, mL 10.00 VII. Analyzing Your Diluted Bleaching Solution Sample determination 0.1050 0.1050 (10) molarity of Na,S,O, solution, M 25.00 25.00 (11) volume of diluted bleaching solution titrated, ml. 44.10 44.30 (12) final buret reading, mL (13) initial buret reading, mL 0.00 0.14 (14) volume of Na,S,O3 solution used, mL (15) number of moles of S2O,2-ion required for titration, mol (16) number of moles of I2 produced in the titration mixture, mol (17) number of moles of OCI ion in diluted bleaching solution titrated, mol (18) mass of NAOC1 present in diluted bleaching solution titrated, g
Data Sheet II. Delivering Solution from Your Buret 17.28 (1) final buret reading, mL (2) initial buret reading, mL 6.52 (3) approved by 10.76 (4) volume of H;O delivered, mL (5) approved by VI. Preparing Your Bleaching Solution Sample Clorox (6) brand name of commercial bleaching solution 4.42 (7) cost of commercial bleaching solution per gallon (see laboratory instructor) 1.12 (8) density of commercial bleaching solution, g mL- (see instructor) (9) volume of commercial bleaching solution diluted to 100 mL, mL 10.00 VII. Analyzing Your Diluted Bleaching Solution Sample determination 0.1050 0.1050 (10) molarity of Na,S,O, solution, M 25.00 25.00 (11) volume of diluted bleaching solution titrated, ml. 44.10 44.30 (12) final buret reading, mL (13) initial buret reading, mL 0.00 0.14 (14) volume of Na,S,O3 solution used, mL (15) number of moles of S2O,2-ion required for titration, mol (16) number of moles of I2 produced in the titration mixture, mol (17) number of moles of OCI ion in diluted bleaching solution titrated, mol (18) mass of NAOC1 present in diluted bleaching solution titrated, g
Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 1P
Related questions
Question

Transcribed Image Text:(19) volume of commercial bleaching solution titrated, mL
(20) mass of commercial bleaching solution titrated, g
(21) percent NaOCI in commercial bleaching solution, %
(22) mean percent NaOCl in commercial bleaching
solution, %
number of moles of
(volume of Na2S2O3
IL
S20,2- ion required, mol
solution, mL
1000 mL
(number of moles of NazS2O3\/1 mol S2O,2-ion
volume of solution, L
15
1 mol NazS2O3
number of moles of
16
1 mol I2
S2O32- ion required, mol/2 mol S2O, ion,
number of moles of
Iz produced, mol
I mol OCI ion'
1 mol I2
number of moles
17 number of moles of OCI ion present, mol =
= (of 2 produced, mol
mass of NaOCl in titrated
18
bleaching solution, g
1 mol NaOCl
1 mol OCI ion/1 mol NaOCI
number of moles of
(74.44 g NaOCI
%3D
OCI" ion present, mol
volume of
commercial bleaching
solution diluted, mL
total volume
of diluted bleaching
solution, mL
volume of diluted
volume of commercial bleaching
19
bleaching solution
titrated, mL
solution titrated, mL
mass of solution, g
-1
20 density, g mL
volume of solution, mL
mass of NaOCI, g
mass of bleaching solution, g,
21
percent NaOCl in bleaching solution, % =
(100%)
percent NaOCl from
determination 1
percent NaOCI from
determination 2
22
mean percent NaOCI, % =
2
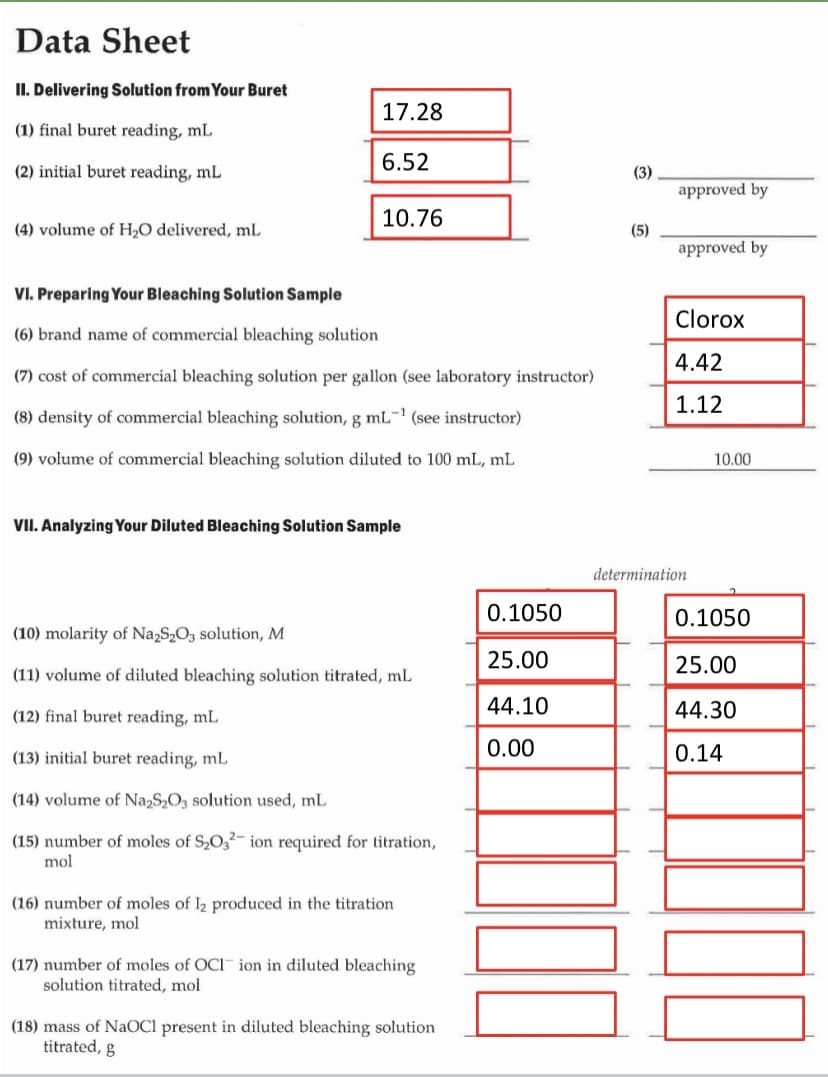
Transcribed Image Text:Data Sheet
II. Delivering Solution from Your Buret
17.28
(1) final buret reading, mL
6.52
(2) initial buret reading, mL
(3)
approved by
10.76
(4) volume of H2O delivered, mL
(5)
approved by
VI. Preparing Your Bleaching Solution Sample
Clorox
(6) brand name of commercial bleaching solution
4.42
(7) cost of commercial bleaching solution per gallon (see laboratory instructor)
1.12
(8) density of commercial bleaching solution, g mL- (see instructor)
(9) volume of commercial bleaching solution diluted to 100 mL, mL
10.00
VII. Analyzing Your Diluted Bleaching Solution Sample
determination
0.1050
0.1050
(10) molarity of Na,S2O3 solution, M
25.00
25.00
(11) volume of diluted bleaching solution titrated, mL
44.10
44.30
(12) final buret reading, mL
(13) initial buret reading, mL
0.00
0.14
(14) volume of Na,S2O3 solution used, mL
(15) number of moles of S,O32- ion required for titration,
mol
(16) number of moles of I2 produced in the titration
mixture, mol
(17) number of moles of OCI ion in diluted bleaching
solution titrated, mol
(18) mass of NaOCl present in diluted bleaching solution
titrated, g
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

