Data Table mass unknown sample (g) volume HCl (mL) molarity HCl (mol/L) 0.5007 25.00 12.1 Write a balanced chemical equation for the reaction between NaHCO; and HCI. NaHCO3 (s) + Hc((ag) → H2CO3 (ag) + NaCl (ag) Calculate the moles of HCl by multiplying the volume of HCl in liters and the molarity of HCl in mol/L. (Keep four significant digits in all of the calculations.) 0.3025 moles 5. The moles of HCl can be converted to moles of NAHCO; using the coefficients from the balanced equation. What is the mole to mole ratio of HCl to NaHCO;? How many moles of NaHCO; are present in the sample?
Data Table mass unknown sample (g) volume HCl (mL) molarity HCl (mol/L) 0.5007 25.00 12.1 Write a balanced chemical equation for the reaction between NaHCO; and HCI. NaHCO3 (s) + Hc((ag) → H2CO3 (ag) + NaCl (ag) Calculate the moles of HCl by multiplying the volume of HCl in liters and the molarity of HCl in mol/L. (Keep four significant digits in all of the calculations.) 0.3025 moles 5. The moles of HCl can be converted to moles of NAHCO; using the coefficients from the balanced equation. What is the mole to mole ratio of HCl to NaHCO;? How many moles of NaHCO; are present in the sample?
Chapter4: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 141IP: In a 1-L beaker, 203 mL of 0.307 M ammonium chromate was mixed with 137 mL of 0.269 M chromium(III)...
Related questions
Question
100%
Please answer question 5
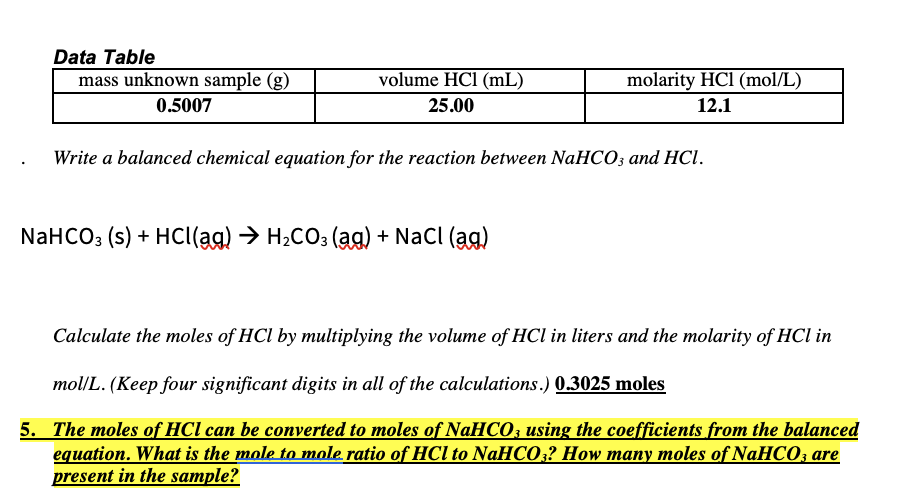
Transcribed Image Text:Data Table
mass unknown sample (g)
volume HCl (mL)
molarity HCl (mol/L)
0.5007
25.00
12.1
Write a balanced chemical equation for the reaction between NaHCO; and HCl.
NaHCO3 (s) + Hc((ag) → H2CO3 (ag) + NaCl (ag)
Calculate the moles of HCl by multiplying the volume of HCl in liters and the molarity of HCl in
mol/L. (Keep four significant digits in all of the calculations.) 0.3025 moles
5. The moles of HCl can be converted to moles of NAHCO; using the coefficients from the balanced
equation. What is the mole to mole ratio of HCl to NaHCO;? How many moles of NaHCO, are
present in the sample?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT