Determine the volume of occupied by 0.053 moles of carbon dioxide gas at 0.0 degrees Celcius at 1 atm. . Hint: Use ideal gas equation: pv=nRT. The R values are provided below: * Ideal Gas Law The value of R depends on the pressure units: Value of R Units of Pressure Volume = Liter 0.0821 atm Temp = Kelvin 8.314 kPa n= moles 62.4 mm Hg ; torr
Determine the volume of occupied by 0.053 moles of carbon dioxide gas at 0.0 degrees Celcius at 1 atm. . Hint: Use ideal gas equation: pv=nRT. The R values are provided below: * Ideal Gas Law The value of R depends on the pressure units: Value of R Units of Pressure Volume = Liter 0.0821 atm Temp = Kelvin 8.314 kPa n= moles 62.4 mm Hg ; torr
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter9: The Gaseous State
Section: Chapter Questions
Problem 23P
Related questions
Question
question 16
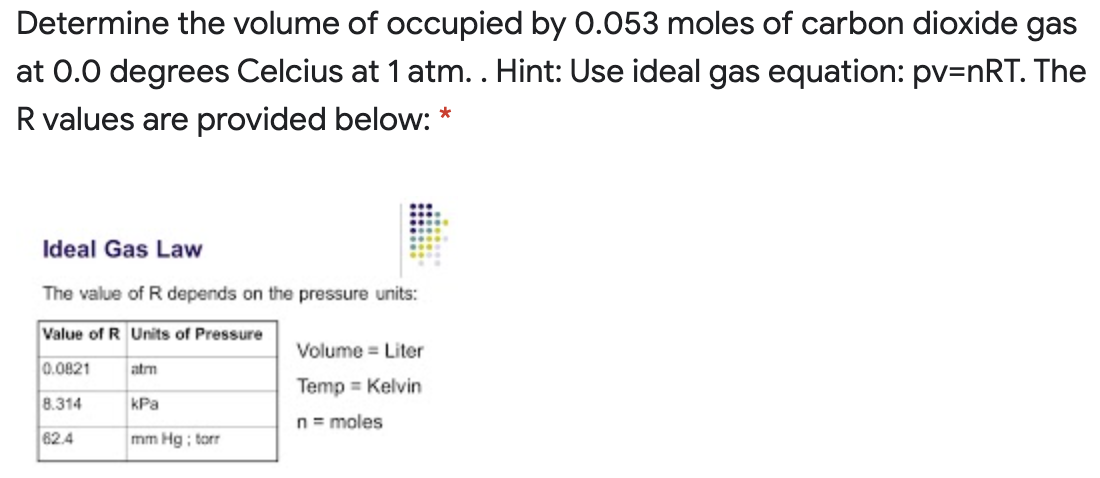
Transcribed Image Text:Determine the volume of occupied by 0.053 moles of carbon dioxide gas
at 0.0 degrees Celcius at 1 atm. . Hint: Use ideal gas equation: pv=nRT. The
R values are provided below: *
Ideal Gas Law
The value of R depends on the pressure units:
Value of R Units of Pressure
Volume = Liter
0.0821
atm
Temp = Kelvin
8.314
kPa
n= moles
62.4
mm Hg ; torr
Expert Solution
Step 1
According to Ideal equation PV=nRT
where P is pressure, V= volume, n = number of moles , R= Ideal gas constant 0.0821 lit.atm./mol.kelvin, T= Temperature
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning