Directions: Complete the data table below. It is not necessary to construct any models. Chemical Valence Number of bonds & number of lone pairs around central atom name Formula electrons Lewis structure NCI, N-5 3 bonds Nitrogen C- 7(3) 20 lone pairs trichloride Sis, Si-4 Silicon S-6(2) disulfide Н-1 C-4 N-5 HCN
Directions: Complete the data table below. It is not necessary to construct any models. Chemical Valence Number of bonds & number of lone pairs around central atom name Formula electrons Lewis structure NCI, N-5 3 bonds Nitrogen C- 7(3) 20 lone pairs trichloride Sis, Si-4 Silicon S-6(2) disulfide Н-1 C-4 N-5 HCN
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter5: Resonance
Section: Chapter Questions
Problem 6CTQ
Related questions
Question
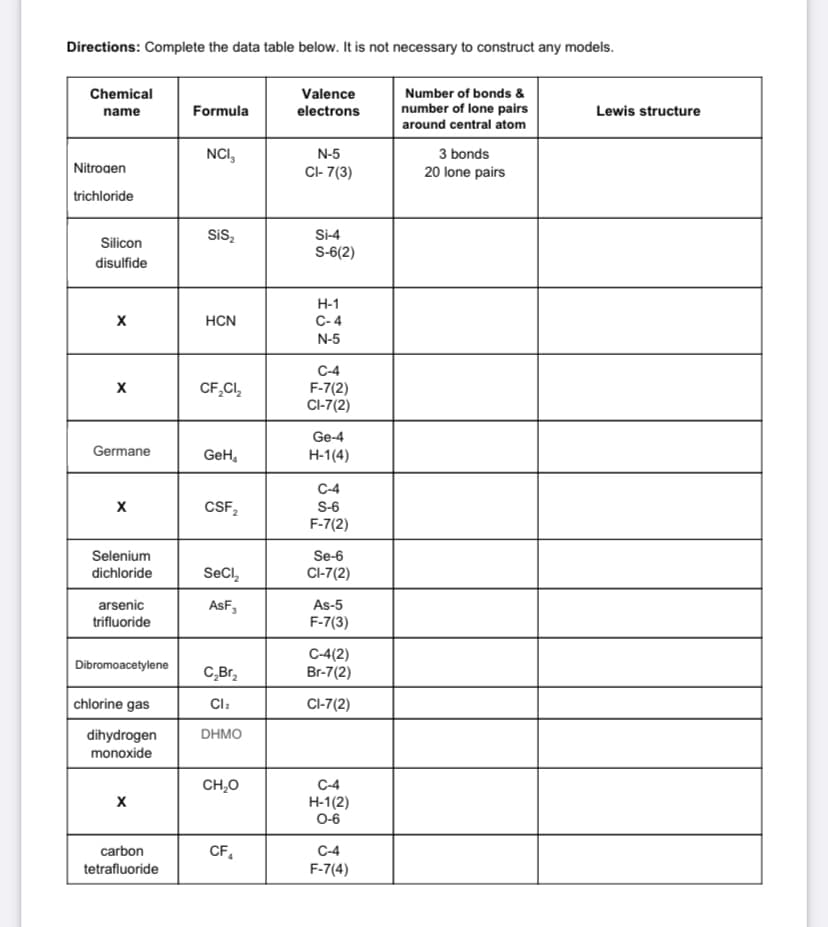
Transcribed Image Text:Directions: Complete the data table below. It is not necessary to construct any models.
Number of bonds &
number of lone pairs
around central atom
Chemical
Valence
electrons
name
Formula
Lewis structure
NCI,
N-5
3 bonds
Nitrogen
CI- 7(3)
20 lone pairs
trichloride
Si-4
S-6(2)
Sis,
Silicon
disulfide
Н-1
C- 4
HCN
N-5
C-4
CF,CI,
F-7(2)
CI-7(2)
Ge-4
Germane
GeH,
H-1(4)
C-4
CSF,
S-6
F-7(2)
Selenium
Se-6
dichloride
SeCl,
CI-7(2)
AsF,
As-5
F-7(3)
arsenic
trifluoride
C-4(2)
Br-7(2)
Dibromoacetylene
chlorine gas
Cl:
CI-7(2)
dihydrogen
monoxide
DHMO
CH,O
C-4
H-1(2)
O-6
carbon
CF,
C-4
tetrafluoride
F-7(4)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,
