Do It Exercise 1: In the adjacent setup, iron is added to acid in a flask fitted with empty balloon. The mass of the setup is noted using digital balance. During reaction hydrogen gas and salt are produced. 325.6 g 325.6 B 325.6 8 1- What is the evidence for this reaction? During Reaction At End of Reaction Before Reaction chemix.org 2- Based on the figure, circle the correct answers and fill the masses in the last row: During Reaction Acid – Before Reaction At end of reaction Substances in Acid – Iron – Iron Acid - Iron – salt - hydrogen Empty – Filled – semi filled salt - hydrogen Empty - Filled – semi filled salt - hydrogen Empty - Filled – semi filled flask & balloon Balloon Mass of setup 3- Compare the masses before, during and at end of reaction. What do you conclude? 4- Write the word equation of the reaction.
Do It Exercise 1: In the adjacent setup, iron is added to acid in a flask fitted with empty balloon. The mass of the setup is noted using digital balance. During reaction hydrogen gas and salt are produced. 325.6 g 325.6 B 325.6 8 1- What is the evidence for this reaction? During Reaction At End of Reaction Before Reaction chemix.org 2- Based on the figure, circle the correct answers and fill the masses in the last row: During Reaction Acid – Before Reaction At end of reaction Substances in Acid – Iron – Iron Acid - Iron – salt - hydrogen Empty – Filled – semi filled salt - hydrogen Empty - Filled – semi filled salt - hydrogen Empty - Filled – semi filled flask & balloon Balloon Mass of setup 3- Compare the masses before, during and at end of reaction. What do you conclude? 4- Write the word equation of the reaction.
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.19QAP
Related questions
Question
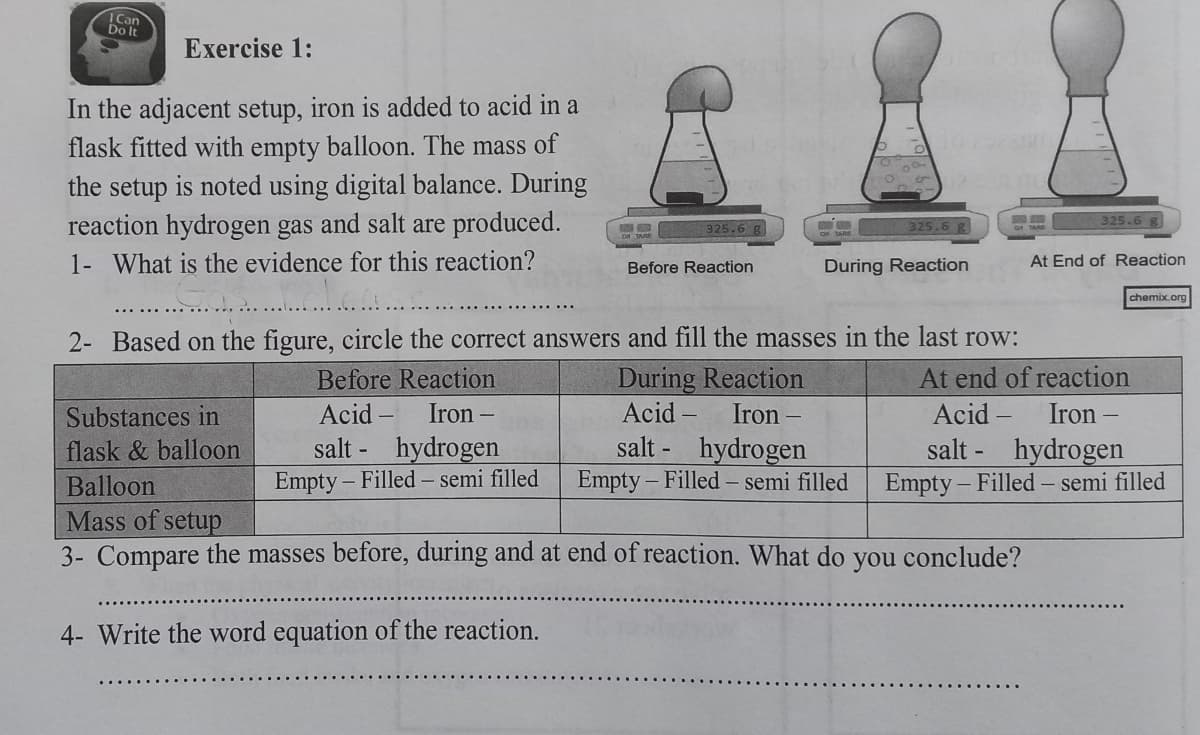
Transcribed Image Text:Can
Do It
Exercise 1:
In the adjacent setup, iron is added to acid in a
flask fitted with empty balloon. The mass of
the setup is noted using digital balance. During
reaction hydrogen gas and salt are produced.
325.6 g
325.6 g
325.6 g
1- What is the evidence for this reaction?
During Reaction
At End of Reaction
Before Reaction
chemix.org
2- Based on the figure, circle the correct answers and fill the masses in the last row:
During Reaction
Acid
Before Reaction
At end of reaction
Substances in
Acid -
Iron –
Iron
Acid -
Iron -
salt - hydrogen
hydrogen
- Filled- semi filled
salt - hydrogen
Empty - Filled – semi filled
flask & balloon
salt -
Balloon
Empty
Empty -
- Filled - semi filled
Mass of setup
3- Compare the masses before, during and at end of reaction. What do you conclude?
4- Write the word equation of the reaction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning