Double Replacement Aqueous sodium hydroxide and hydrochloric acid Page 3 of 5 Chemistry 19 - Santa Monica College Double Replacement followed by Decomposition The product of the double displacement spontaneously decomposes into H,O and CO, Solid sodium bicarbonate and hydrochloric acid 6 Double Replacement Aqueous iron(III) chloride and aqueous ammonium hydroxide 7 Double Replacement Aqueous sodium chloride and aqueous potassium nitrate
Double Replacement Aqueous sodium hydroxide and hydrochloric acid Page 3 of 5 Chemistry 19 - Santa Monica College Double Replacement followed by Decomposition The product of the double displacement spontaneously decomposes into H,O and CO, Solid sodium bicarbonate and hydrochloric acid 6 Double Replacement Aqueous iron(III) chloride and aqueous ammonium hydroxide 7 Double Replacement Aqueous sodium chloride and aqueous potassium nitrate
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter5: Chemical Reactions
Section: Chapter Questions
Problem 5.90E
Related questions
Question
100%
Fill in for parts 4,5,6,7
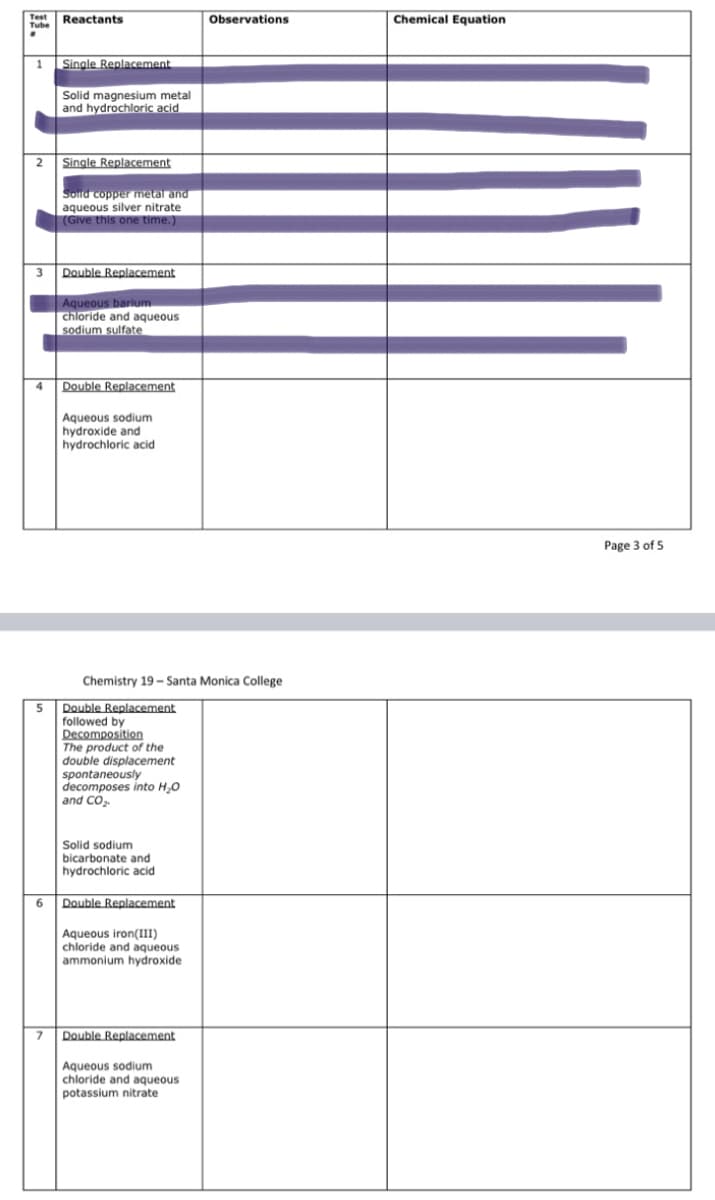
Transcribed Image Text:Chemical Equation
Test
Observations
Tube Reactants
1 Single Replacement
Solid magnesium metal
and hydrochloric acid
2 Single Replacement
Solld copper metal and
aqueous silver nitrate
(Give this one time.)
3 Double Replacement
Aqueous barium
chloride and aqueous
sodium sulfate
Double Replacement
4
Aqueous sodium
hydroxide and
hydrochloric acid
Page 3 of 5
Chemistry 19 - Santa Monica College
5 Double Replacement
followed by
Decomposition
The product of the
double displacement
spontaneously
decomposes into H,0
and CO,
Solid sodium
bicarbonate and
hydrochloric acid
6.
Double Replacement
Aqueous iron(III)
chloride and aqueous
ammonium hydroxide
7 Double Replacement
Aqueous sodium
chloride and aqueous
potassium nitrate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry In Focus
Chemistry
ISBN:
9781305084476
Author:
Tro, Nivaldo J., Neu, Don.
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry In Focus
Chemistry
ISBN:
9781305084476
Author:
Tro, Nivaldo J., Neu, Don.
Publisher:
Cengage Learning