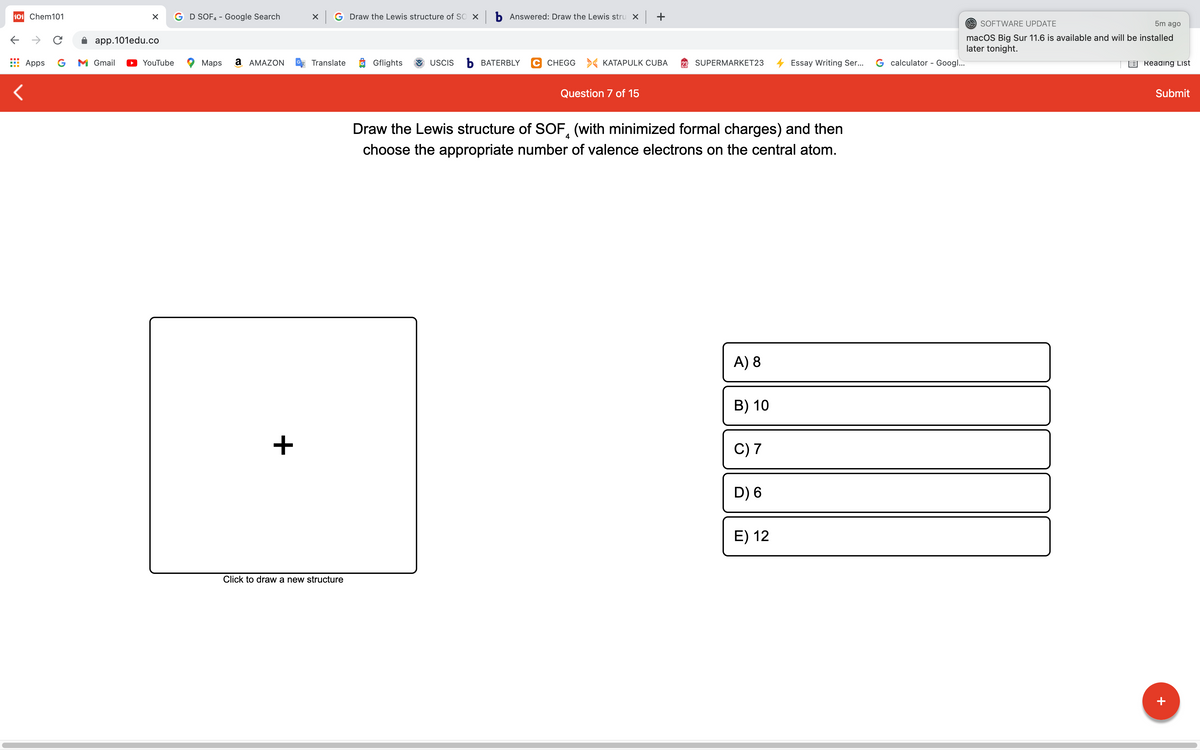
Draw the Lewis structure of SOF, (with minimized formal charges) and then choose the appropriate number of valence electrons on the central atom. A) 8 B) 10 + C) 7 D) 6 E) 12 Click to draw a new structure
Draw the Lewis structure of SOF, (with minimized formal charges) and then choose the appropriate number of valence electrons on the central atom. A) 8 B) 10 + C) 7 D) 6 E) 12 Click to draw a new structure
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter10: Molecular Structure And Bonding Theories
Section: Chapter Questions
Problem 10.43QE: For each of the following molecules, complete the Lewis structure and use the VSEPR model to...
Related questions
Question
Transcribed Image Text:101 Chem101
G D SOF4 - Google Search
G Draw the Lewis structure of SO X b Answered: Draw the Lewis stru x +
O SOFTWARE UPDATE
5m ago
macOS Big Sur 11.6 is available and will be installed
later tonight.
app.101edu.co
Apps
G
M Gmail
YouTube
Maps
а АМAZON
Translate
O Gflights
USCIS
Ь ВАТERBLY
C CHEGG
> KATAPULK CUBA
SUPERMARKET23
Essay Writing Ser..
G calculator - Googl...
E Reading List
Question 7 of 15
Submit
Draw the Lewis structure of SOF, (with minimized formal charges) and then
4
choose the appropriate number of valence electrons on the central atom.
A) 8
B) 10
+
C) 7
D) 6
E) 12
Click to draw a new structure
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax