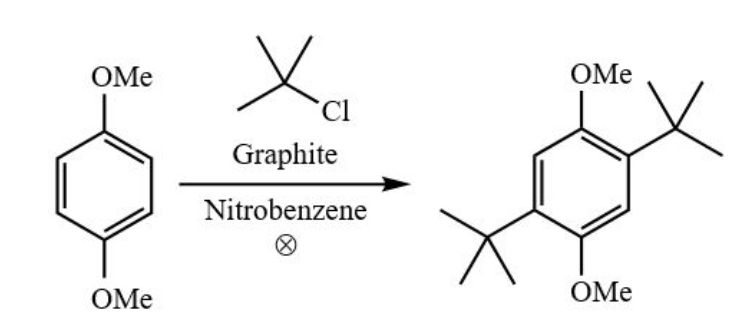
0.56 g 1,4‐dimethoxybenzene, 0.9 mL 2‐chloro‐2‐methylpropane, 5 mL nitrobenzene and 0.52 g graphite were combined, and the mixture was refluxed for 1.5 hours. It was then cooled to room temperature and filtered. The filtered graphite was washed with 15 mL hexane. The filtrate was distilled under vacuum to remove any remaining 1,4‐dimethoxybenzene, 2‐chloro‐2‐ methylpropane and nitrobenzene (6.3 g), yielding 0.44 g 1,4‐di‐tert‐butyl‐2,5‐dimethoxybenzene (43.4% yield). What is the Atom economy, E factor, and effective mass yield?
0.56 g 1,4‐dimethoxybenzene, 0.9 mL 2‐chloro‐2‐methylpropane, 5 mL nitrobenzene and 0.52 g graphite were combined, and the mixture was refluxed for 1.5 hours. It was then cooled to room temperature and filtered. The filtered graphite was washed with 15 mL hexane. The filtrate was distilled under vacuum to remove any remaining 1,4‐dimethoxybenzene, 2‐chloro‐2‐ methylpropane and nitrobenzene (6.3 g), yielding 0.44 g 1,4‐di‐tert‐butyl‐2,5‐dimethoxybenzene (43.4% yield). What is the Atom economy, E factor, and effective mass yield?
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter1: Chemistry: An Introduction
Section1.1: The Science Of Chemistry
Problem 3RQ
Related questions
Question
0.56 g 1,4‐dimethoxybenzene, 0.9 mL 2‐chloro‐2‐methylpropane, 5 mL nitrobenzene and 0.52 g graphite were combined, and the mixture was refluxed for 1.5 hours. It was then cooled to room temperature and filtered. The filtered graphite was washed with 15 mL hexane. The filtrate was distilled under vacuum to remove any remaining 1,4‐dimethoxybenzene, 2‐chloro‐2‐ methylpropane and nitrobenzene (6.3 g), yielding 0.44 g 1,4‐di‐tert‐butyl‐2,5‐dimethoxybenzene (43.4% yield). What is the Atom economy, E factor, and effective mass yield?
Transcribed Image Text:OMe
OMe
Cl
Graphite
Nitrobenzene
OMe
OMe
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER