+ e Home ourse.html?courseld=15539865&HepID=676f177415469ec3dc78ff3c8 bf724d9#1 0001 Inbox-Outlook We... S Sugarpill Cosmetic... Shop | Origami Owl Wholesale Glass M... S The Corinthian Hou... zed Invitat... HW < 12 of 16 se 10.31 Constants Periodic Table Part A Calculate the final Celsius temperature of sulfur dioxide gas if 50.0 mL of the gas at 25 °C and 0.505 atm is heated until the pressure is 0.705 atm. Assume that the volume remains constant. Express your answer using two significant figures. VO AD ΑΣΦ ? T = Request Answer Submit Next> Provide Feedback P Pearson Contact Us Copyright 2019 Pearson Education Inc. All rights reserved. Terms of Use Privacy Policy | Permissions MacBook Air »)) DD DII O00 F12 F11 F10 F9 F8 F7 F4 F6 F5 F3 + $ & 3 0 9 4 7 8 6 5 } { P E U R Y D L K G F H T 2LO
+ e Home ourse.html?courseld=15539865&HepID=676f177415469ec3dc78ff3c8 bf724d9#1 0001 Inbox-Outlook We... S Sugarpill Cosmetic... Shop | Origami Owl Wholesale Glass M... S The Corinthian Hou... zed Invitat... HW < 12 of 16 se 10.31 Constants Periodic Table Part A Calculate the final Celsius temperature of sulfur dioxide gas if 50.0 mL of the gas at 25 °C and 0.505 atm is heated until the pressure is 0.705 atm. Assume that the volume remains constant. Express your answer using two significant figures. VO AD ΑΣΦ ? T = Request Answer Submit Next> Provide Feedback P Pearson Contact Us Copyright 2019 Pearson Education Inc. All rights reserved. Terms of Use Privacy Policy | Permissions MacBook Air »)) DD DII O00 F12 F11 F10 F9 F8 F7 F4 F6 F5 F3 + $ & 3 0 9 4 7 8 6 5 } { P E U R Y D L K G F H T 2LO
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Transcribed Image Text:+
e Home
ourse.html?courseld=15539865&HepID=676f177415469ec3dc78ff3c8 bf724d9#1 0001
Inbox-Outlook We...
S Sugarpill Cosmetic...
Shop | Origami Owl
Wholesale Glass M...
S The Corinthian Hou...
zed Invitat...
HW
<
12 of 16
se 10.31
Constants Periodic Table
Part A
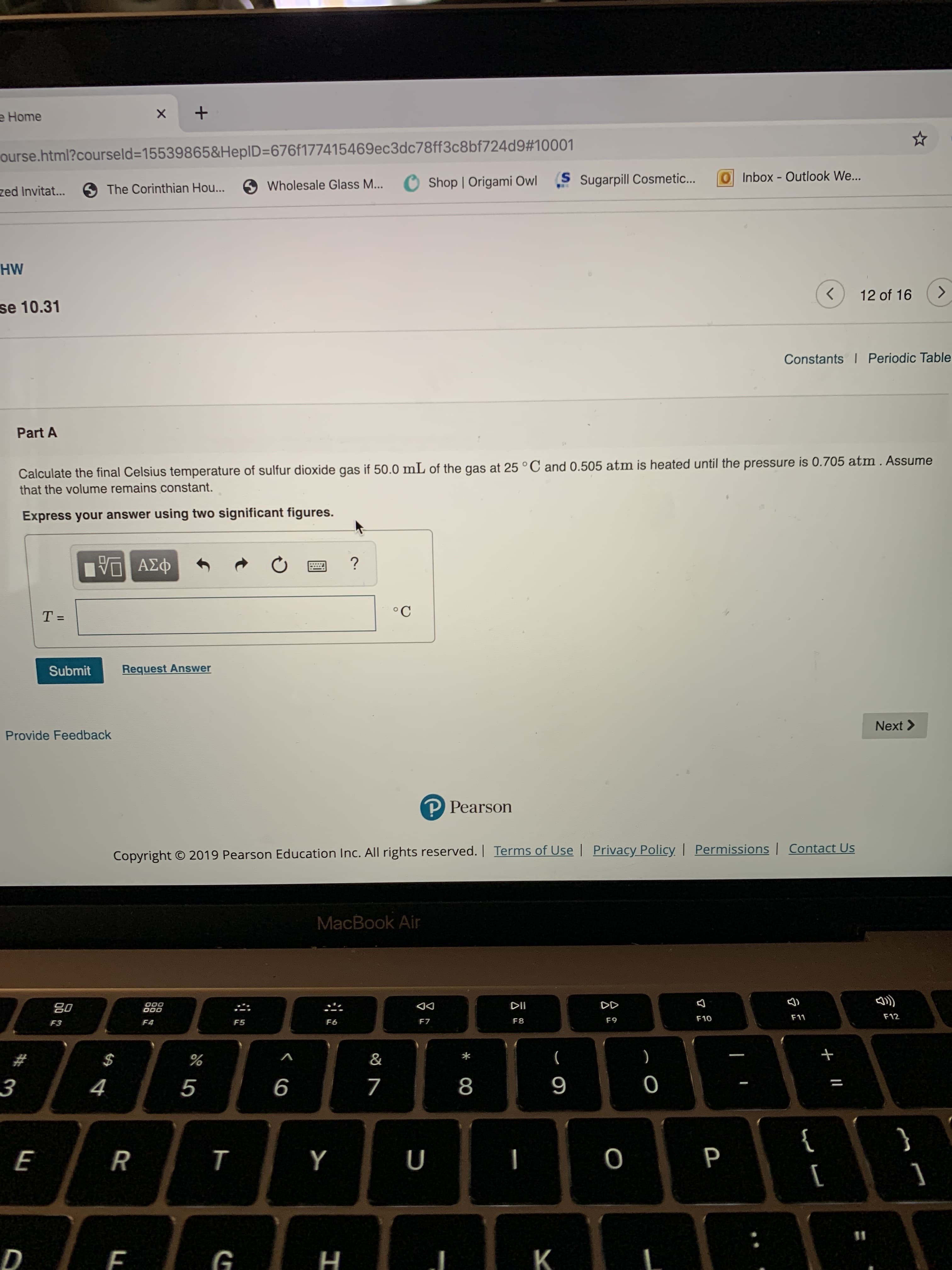
Calculate the final Celsius temperature of sulfur dioxide gas if 50.0 mL of the gas at 25 °C and 0.505 atm is heated until the pressure is 0.705 atm. Assume
that the volume remains constant.
Express your answer using two significant figures.
VO AD
ΑΣΦ
?
T =
Request Answer
Submit
Next>
Provide Feedback
P Pearson
Contact Us
Copyright 2019 Pearson Education Inc. All rights reserved. Terms of Use Privacy Policy | Permissions
MacBook Air
»))
DD
DII
O00
F12
F11
F10
F9
F8
F7
F4
F6
F5
F3
+
$
&
3
0
9
4
7
8
6
5
}
{
P
E
U
R
Y
D
L
K
G
F
H
T
2LO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY