E17E.1(b) The reaction mechanism for renaturation of a double helix from its strands A and B is thought to be A+B U U -, H where U is an unstable helix, and H is the stable form of the helix. The reaction between A and B is first order in each species and the return of U to A + B is first order in U; the reaction of U to H is first order in U. Deduce the rate law for the rate of formation of H in two ways: (i) by assuming a pre-equilibrium and (ii) by assuming that the steady-state approximation can be applied to U.
E17E.1(b) The reaction mechanism for renaturation of a double helix from its strands A and B is thought to be A+B U U -, H where U is an unstable helix, and H is the stable form of the helix. The reaction between A and B is first order in each species and the return of U to A + B is first order in U; the reaction of U to H is first order in U. Deduce the rate law for the rate of formation of H in two ways: (i) by assuming a pre-equilibrium and (ii) by assuming that the steady-state approximation can be applied to U.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter11: Rate Of Reaction
Section: Chapter Questions
Problem 53QAP: Ammonium cyanate, NH4NCO, in water rearranges to produce urea, a common fertilizer, (NH2)2CO:...
Related questions
Question
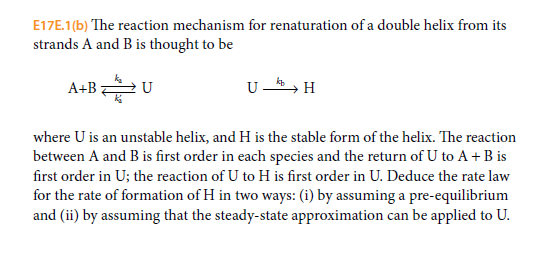
Transcribed Image Text:E17E.1(b) The reaction mechanism for renaturation of a double helix from its
strands A and B is thought to be
A+B U
U -, H
where U is an unstable helix, and H is the stable form of the helix. The reaction
between A and B is first order in each species and the return of U to A + B is
first order in U; the reaction of U to H is first order in U. Deduce the rate law
for the rate of formation of H in two ways: (i) by assuming a pre-equilibrium
and (ii) by assuming that the steady-state approximation can be applied to U.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax