Each of the insoluble salts below are put into 0.10 M hydrochloric acid solution. Do you expect their solubility to be more, less, or about the same as in a pure water solution ? Clear All More soluble than in pure silver chloride water. Similar solubility as in pure chromium(III) hydroxide water. Less soluble than in pure iron(III) sulfide water.
Each of the insoluble salts below are put into 0.10 M hydrochloric acid solution. Do you expect their solubility to be more, less, or about the same as in a pure water solution ? Clear All More soluble than in pure silver chloride water. Similar solubility as in pure chromium(III) hydroxide water. Less soluble than in pure iron(III) sulfide water.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter16: Solubility And Precipitation Equilibria
Section: Chapter Questions
Problem 82CP
Related questions
Question
100%
9
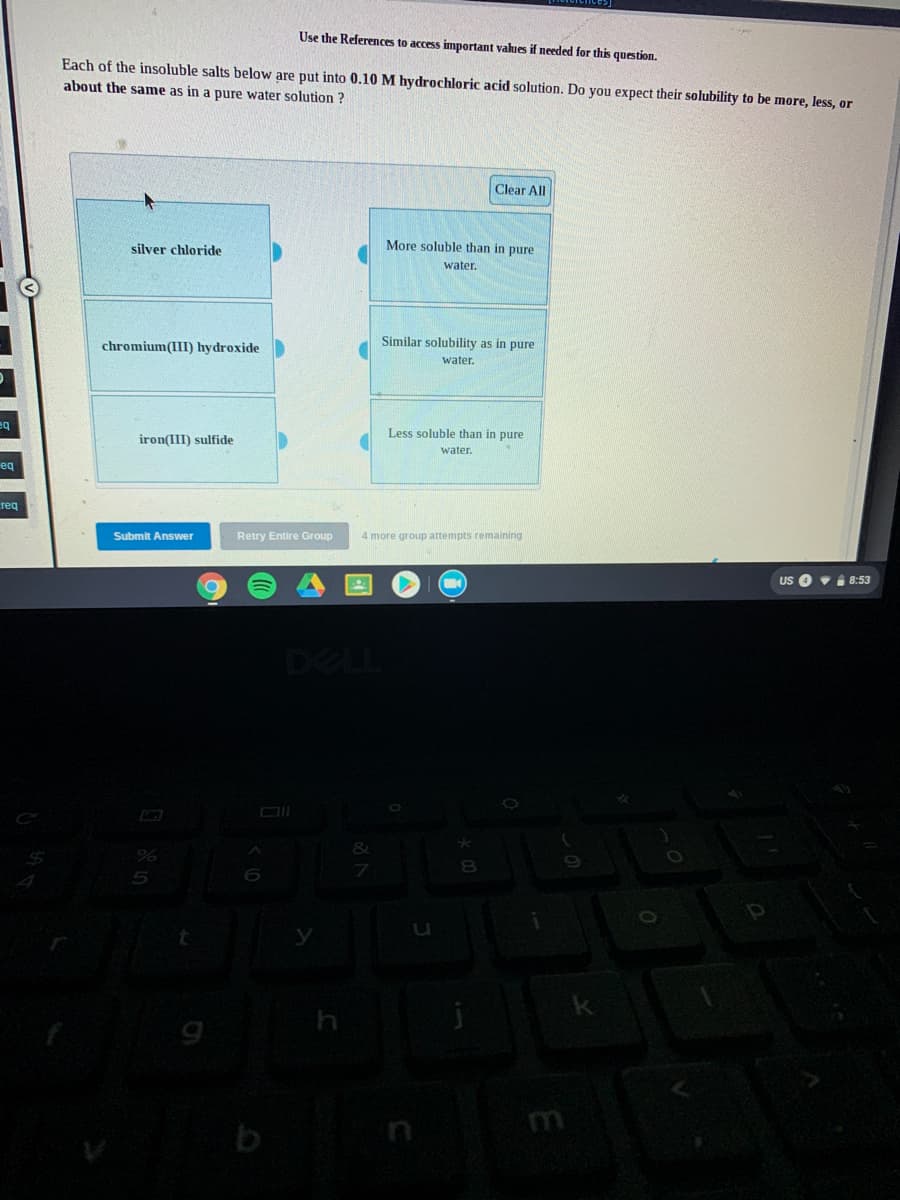
Transcribed Image Text:Use the References to access important values if needed for this question.
Each of the insoluble salts below are put into 0.10 M hydrochloric acid solution. Do you expect their solubility to be more, less, or
about the same as in a pure water solution ?
Clear All
silver chloride
More soluble than in pure
water.
Similar solubility as in pure
chromium(III) hydroxide
water.
eq
Less soluble than in pure
iron(III) sulfide
water.
eq
req
Submit Answer
Retry Entire Group
4 more group attempts remaining
US
i 8:53
DELL
%24
n
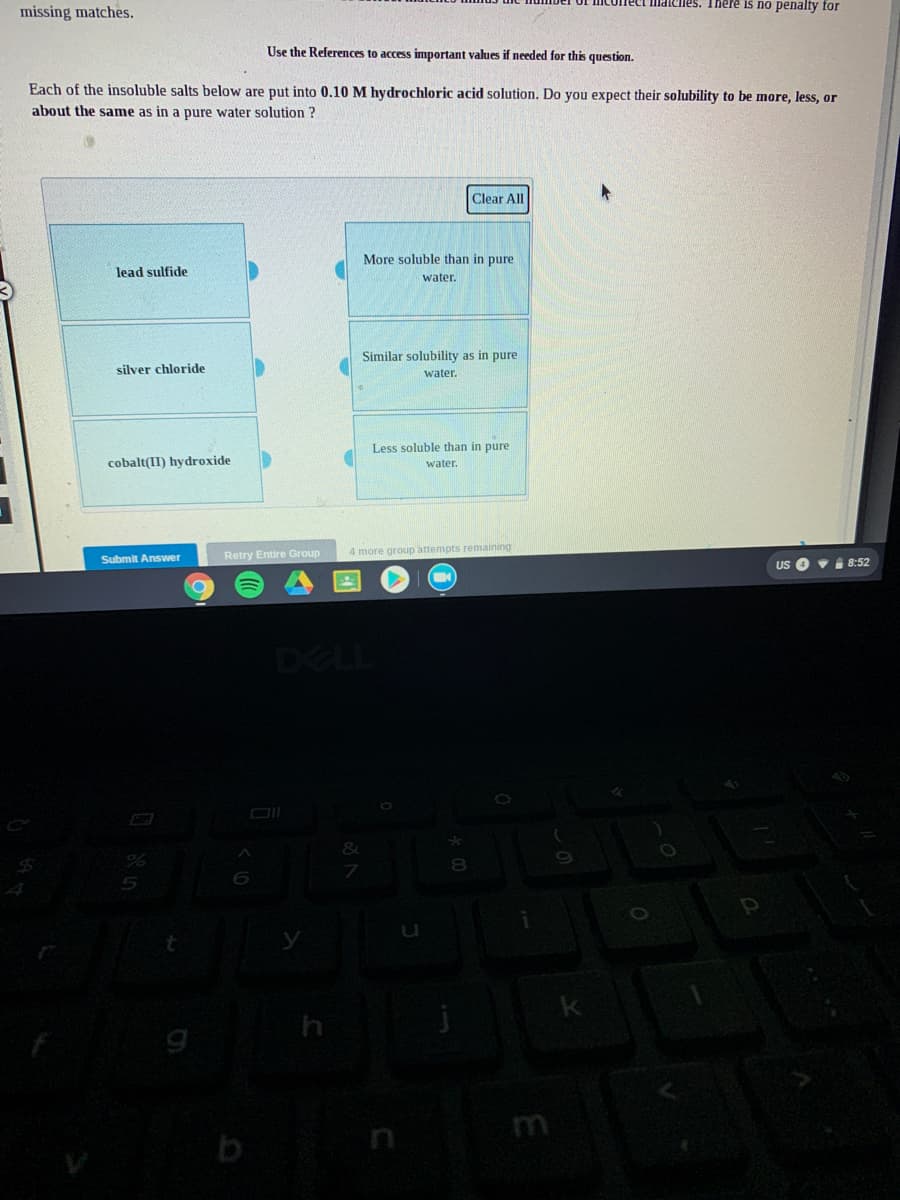
Transcribed Image Text:Ilmatciles. There is no penalty for
missing matches.
Use the References to access important values if needed for this question.
Each of the insoluble salts below are put into 0.10 M hydrochloric acid solution. Do you expect their solubility to be more, less, or
about the same as in a pure water solution ?
Clear All
More soluble than in pure
lead sulfide
water.
Similar solubility as in pure
silver chloride
water.
Less soluble than in pure
cobalt(II) hydroxide
water.
Retry Entire Group
4 more group attempts remaining
Submit Answer
US O •i 8:52
DELL
8.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning



Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co