Enamines can serve as enolate surrogates in reactions at the a-carbon. In the reaction sequence, the structures of the enamine addition product – the initial zwitterion and its neutral tautomer – are shown. Draw the structures of the two reactants forming these intermediates, and draw the structure of the final product, obtained via hydrolysis of the neutral intermediate. initial zwitterionic intermediate neutral intermediate tautomerization Reactants H20 hydrolysis product Draw the two reactants. Draw the hydrolysis product.
Enamines can serve as enolate surrogates in reactions at the a-carbon. In the reaction sequence, the structures of the enamine addition product – the initial zwitterion and its neutral tautomer – are shown. Draw the structures of the two reactants forming these intermediates, and draw the structure of the final product, obtained via hydrolysis of the neutral intermediate. initial zwitterionic intermediate neutral intermediate tautomerization Reactants H20 hydrolysis product Draw the two reactants. Draw the hydrolysis product.
Chapter18: Ethers And Epoxides; Thiols And Sulfides
Section18.SE: Something Extra
Problem 69GP
Related questions
Question
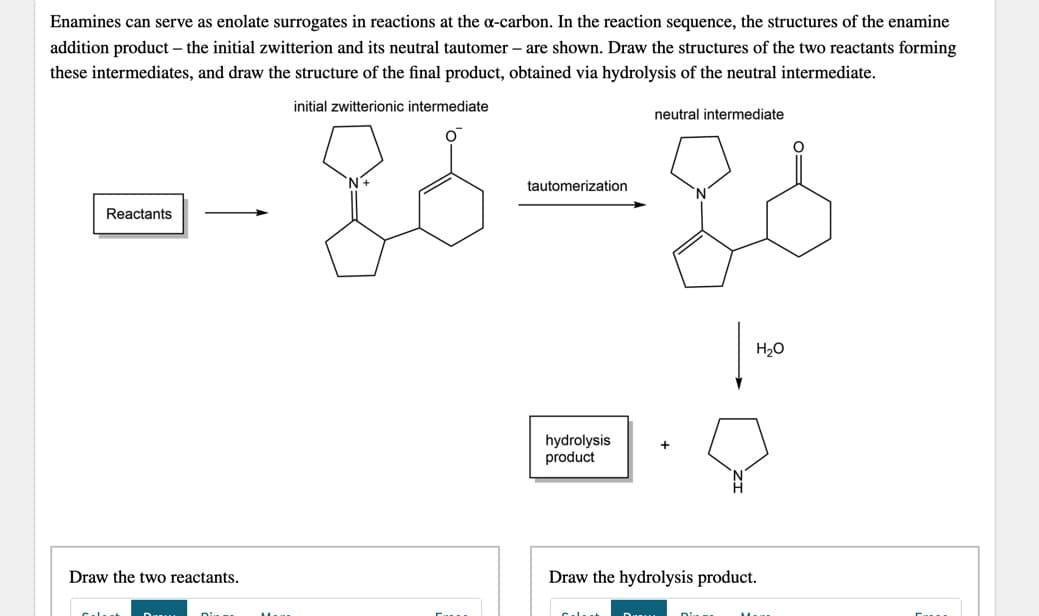
Transcribed Image Text:Enamines can serve as enolate surrogates in reactions at the a-carbon. In the reaction sequence, the structures of the enamine
addition product – the initial zwitterion and its neutral tautomer – are shown. Draw the structures of the two reactants forming
these intermediates, and draw the structure of the final product, obtained via hydrolysis of the neutral intermediate.
initial zwitterionic intermediate
neutral intermediate
tautomerization
Reactants
H20
hydrolysis
product
Draw the two reactants.
Draw the hydrolysis product.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning