Estimate the length of the unit cell edge, a, from the atomic radius of Aluminum (1.43 Á). ΑΣφ cm Submit Previous Answers Request Answer X Incorrect; Try Again; 3 attempts remaining Part D Calculate the density of Aluminum metal. 271 alem3 Ch. 2 - Chemistr.pdf * Ch. 2 Note Outlin.pdf
Estimate the length of the unit cell edge, a, from the atomic radius of Aluminum (1.43 Á). ΑΣφ cm Submit Previous Answers Request Answer X Incorrect; Try Again; 3 attempts remaining Part D Calculate the density of Aluminum metal. 271 alem3 Ch. 2 - Chemistr.pdf * Ch. 2 Note Outlin.pdf
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter9: Liquids And Solids
Section: Chapter Questions
Problem 60QAP: Aluminum metal crystallizes with a face-centered cubic unit cell. The volume of the cell is 0.0662...
Related questions
Question
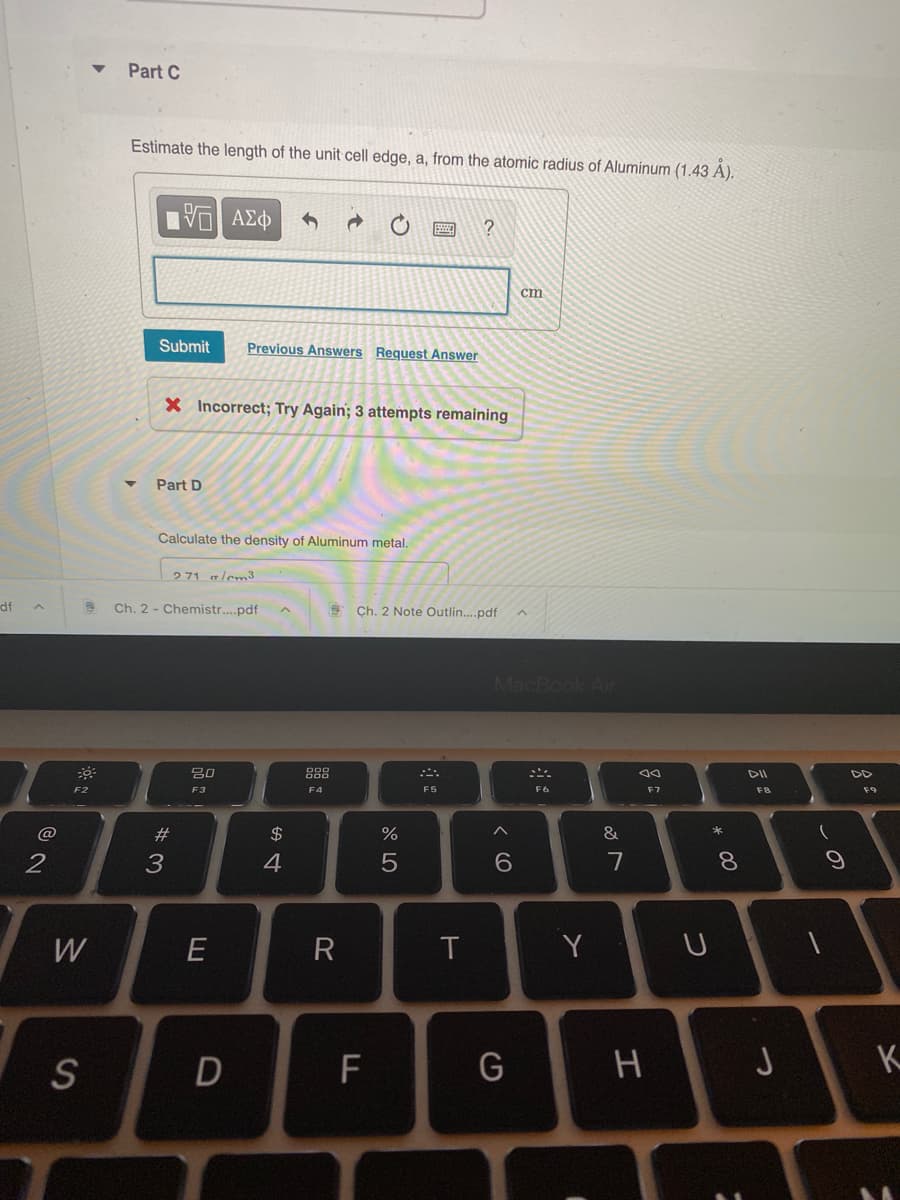
Transcribed Image Text:Part C
Estimate the length of the unit cell edge, a, from the atomic radius of Aluminum (1.43 A).
ΑΣΦ
ст
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 3 attempts remaining
Part D
Calculate the density of Aluminum metal,
271 glem3
df
Ch. 2 - Chemistr.pdf
Ch. 2 Note Outlin..pdf
MacBook Air
吕0
DII
DD
F3
F4
F5
F6
F7
F9
2$
&
3
4
7
8.
W
E
R
Y
S
F
K
この
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning